当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and biological evaluation of new 1,4‐diarylazetidin‐2‐one derivatives (β‐lactams) as selective cyclooxygenase‐2 inhibitors
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-01-09 , DOI: 10.1002/ardp.201900293 Hadi Arefi 1 , Nima Naderi 2 , Amir B Irani Shemirani 3 , Mina Kiani Falavarjani 3 , Mahsa Azami Movahed 1 , Afshin Zarghi 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-01-09 , DOI: 10.1002/ardp.201900293 Hadi Arefi 1 , Nima Naderi 2 , Amir B Irani Shemirani 3 , Mina Kiani Falavarjani 3 , Mahsa Azami Movahed 1 , Afshin Zarghi 1
Affiliation
|
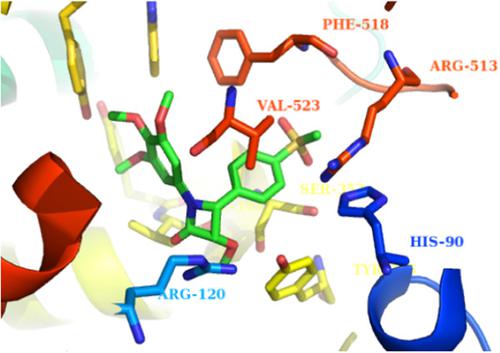
A new series of 1,4‐diarylazetidin‐2‐one derivatives (β‐lactams) were designed and synthesized to evaluate their biological activities as selective cyclooxygenase‐2 (COX‐2) inhibitors. In vitro COX‐1 and COX‐2 inhibition studies showed that all compounds were selective inhibitors of the COX‐2 isozyme with IC50 values in the 0.05–0.11 µM range, and COX‐2 selectivity indexes in the range of 170–703.7. Among the synthesized β‐lactams, 3‐methoxy‐4‐(4‐(methylsulfonyl)phenyl)‐1‐(3,4,5‐trimethoxyphenyl)azetidin‐2‐one (4j) possessing trimethoxy groups at the N‐1 phenyl ring exhibited the highest COX‐2 inhibitory selectivity and potency, even more potent than the reference drug celecoxib. The analgesic activity of the synthesized compounds was also determined using the formalin test. Compound 4f displayed the best analgesic activity among the synthesized molecules. Molecular modeling studies indicated that the methylsulfonyl pharmacophore group can be inserted into the secondary pocket of the COX‐2 active site for interactions with Arg513. The structure–activity data acquired indicate that the β‐lactam ring moiety constitutes a suitable scaffold to design new 1,4‐diarylazetidin‐2‐ones with selective COX‐2 inhibitory activity.
中文翻译:

新型 1,4-二芳基氮杂环丁烷-2-one 衍生物(β-内酰胺类)作为选择性环加氧酶-2 抑制剂的设计、合成和生物学评价
设计并合成了一系列新的 1,4-二芳基氮杂环丁烷-2-one 衍生物(β-内酰胺类),以评估其作为选择性环氧合酶-2(COX-2)抑制剂的生物活性。体外 COX-1 和 COX-2 抑制研究表明,所有化合物都是 COX-2 同工酶的选择性抑制剂,IC50 值在 0.05-0.11 µM 范围内,COX-2 选择性指数在 170-703.7 范围内。在合成的β-内酰胺类中,3-甲氧基-4-(4-(甲基磺酰基)苯基)-1-(3,4,5-三甲氧基苯基)氮杂环丁烷-2-酮(4j)在N-1苯基上具有三甲氧基环表现出最高的 COX-2 抑制选择性和效力,甚至比参考药物塞来昔布更有效。还使用福尔马林试验测定合成化合物的镇痛活性。化合物4f在合成的分子中表现出最好的镇痛活性。分子模型研究表明,甲基磺酰基药效团可以插入到 COX-2 活性位点的二级口袋中,与 Arg513 相互作用。获得的结构-活性数据表明,β-内酰胺环部分构成了一个合适的支架来设计具有选择性 COX-2 抑制活性的新型 1,4-二芳基氮杂环丁烷-2-酮。
更新日期:2020-01-09
中文翻译:

新型 1,4-二芳基氮杂环丁烷-2-one 衍生物(β-内酰胺类)作为选择性环加氧酶-2 抑制剂的设计、合成和生物学评价
设计并合成了一系列新的 1,4-二芳基氮杂环丁烷-2-one 衍生物(β-内酰胺类),以评估其作为选择性环氧合酶-2(COX-2)抑制剂的生物活性。体外 COX-1 和 COX-2 抑制研究表明,所有化合物都是 COX-2 同工酶的选择性抑制剂,IC50 值在 0.05-0.11 µM 范围内,COX-2 选择性指数在 170-703.7 范围内。在合成的β-内酰胺类中,3-甲氧基-4-(4-(甲基磺酰基)苯基)-1-(3,4,5-三甲氧基苯基)氮杂环丁烷-2-酮(4j)在N-1苯基上具有三甲氧基环表现出最高的 COX-2 抑制选择性和效力,甚至比参考药物塞来昔布更有效。还使用福尔马林试验测定合成化合物的镇痛活性。化合物4f在合成的分子中表现出最好的镇痛活性。分子模型研究表明,甲基磺酰基药效团可以插入到 COX-2 活性位点的二级口袋中,与 Arg513 相互作用。获得的结构-活性数据表明,β-内酰胺环部分构成了一个合适的支架来设计具有选择性 COX-2 抑制活性的新型 1,4-二芳基氮杂环丁烷-2-酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号