Nature ( IF 64.8 ) Pub Date : 2020-01-08 , DOI: 10.1038/s41586-019-1882-z Kumiko Yoshioka-Kobayashi 1, 2 , Marina Matsumiya 1, 3 , Yusuke Niino 4 , Akihiro Isomura 1, 5, 6 , Hiroshi Kori 7 , Atsushi Miyawaki 4, 8 , Ryoichiro Kageyama 1, 2, 3, 6
|
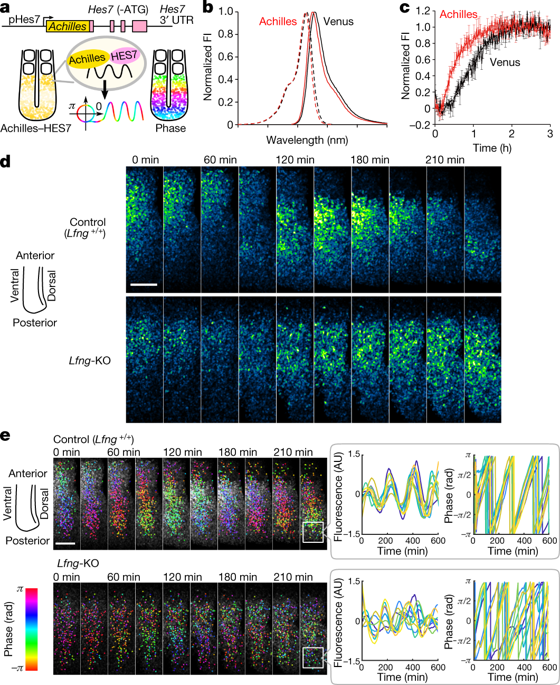
Individual cellular activities fluctuate but are constantly coordinated at the population level via cell–cell coupling. A notable example is the somite segmentation clock, in which the expression of clock genes (such as Hes7) oscillates in synchrony between the cells that comprise the presomitic mesoderm (PSM)1,2. This synchronization depends on the Notch signalling pathway; inhibiting this pathway desynchronizes oscillations, leading to somite fusion3,4,5,6,7. However, how Notch signalling regulates the synchronicity of HES7 oscillations is unknown. Here we establish a live-imaging system using a new fluorescent reporter (Achilles), which we fuse with HES7 to monitor synchronous oscillations in HES7 expression in the mouse PSM at a single-cell resolution. Wild-type cells can rapidly correct for phase fluctuations in HES7 oscillations, whereas the absence of the Notch modulator gene lunatic fringe (Lfng) leads to a loss of synchrony between PSM cells. Furthermore, HES7 oscillations are severely dampened in individual cells of Lfng-null PSM. However, when Lfng-null PSM cells were completely dissociated, the amplitude and periodicity of HES7 oscillations were almost normal, which suggests that LFNG is involved mostly in cell–cell coupling. Mixed cultures of control and Lfng-null PSM cells, and an optogenetic Notch signalling reporter assay, revealed that LFNG delays the signal-sending process of intercellular Notch signalling transmission. These results—together with mathematical modelling—raised the possibility that Lfng-null PSM cells shorten the coupling delay, thereby approaching a condition known as the oscillation or amplitude death of coupled oscillators8. Indeed, a small compound that lengthens the coupling delay partially rescues the amplitude and synchrony of HES7 oscillations in Lfng-null PSM cells. Our study reveals a delay control mechanism of the oscillatory networks involved in somite segmentation, and indicates that intercellular coupling with the correct delay is essential for synchronized oscillation.
中文翻译:

耦合延迟控制分段时钟中的同步振荡
单个细胞活动波动,但通过细胞 - 细胞耦合在群体水平上不断协调。一个值得注意的例子是体节分割时钟,其中时钟基因(如Hes7 )的表达在构成前体中胚层 (PSM) 1,2的细胞之间同步振荡。这种同步依赖于 Notch 信号通路;抑制这条通路会使振荡不同步,导致体节融合3,4,5,6,7. 然而,Notch 信号如何调节 HES7 振荡的同步性尚不清楚。在这里,我们使用新的荧光报告器 (Achilles) 建立了一个实时成像系统,我们将其与 HES7 融合,以单细胞分辨率监测小鼠 PSM 中 HES7 表达的同步振荡。野生型细胞可以快速纠正 HES7 振荡中的相位波动,而 Notch 调节基因疯子边缘 ( Lfng ) 的缺失会导致 PSM 细胞之间失去同步。此外,HES7 振荡在Lfng -null PSM 的单个细胞中受到严重抑制。然而,当Lfng-null PSM 细胞完全解离,HES7 振荡的幅度和周期性几乎正常,这表明 LFNG 主要参与细胞-细胞耦合。对照和Lfng无效 PSM 细胞的混合培养物以及光遗传学 Notch 信号报告基因分析表明,LFNG 延迟了细胞间 Notch 信号传递的信号发送过程。这些结果与数学建模一起提出了Lfng零 PSM 单元缩短耦合延迟的可能性,从而接近称为耦合振荡器8的振荡或振幅死亡的条件。事实上,一种延长耦合延迟的小化合物部分地挽救了 Lfng 中 HES7 振荡的幅度和同步性-null PSM 单元。我们的研究揭示了参与体节分割的振荡网络的延迟控制机制,并表明具有正确延迟的细胞间耦合对于同步振荡至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号