当前位置:
X-MOL 学术
›
Energy Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible High Capacity and Reaction Mechanism of Cr2(NCN)3 Negative Electrodes for Li‐Ion Batteries
Energy Technology ( IF 3.6 ) Pub Date : 2020-01-29 , DOI: 10.1002/ente.201901260 Jeethu Jiju Arayamparambil 1, 2 , Kaixuan Chen 3 , Antonella Iadecola 4 , Markus Mann 3 , Xianji Qiao 3 , Bernard Fraisse 1 , Richard Dronskowski 3, 5 , Lorenzo Stievano 1, 2, 4 , Moulay Tahar Sougrati 1, 2, 4
Energy Technology ( IF 3.6 ) Pub Date : 2020-01-29 , DOI: 10.1002/ente.201901260 Jeethu Jiju Arayamparambil 1, 2 , Kaixuan Chen 3 , Antonella Iadecola 4 , Markus Mann 3 , Xianji Qiao 3 , Bernard Fraisse 1 , Richard Dronskowski 3, 5 , Lorenzo Stievano 1, 2, 4 , Moulay Tahar Sougrati 1, 2, 4
Affiliation
|
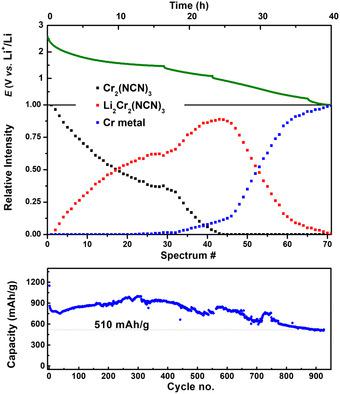
A detailed study of the electrochemical reaction mechanism between lithium and the trivalent transition‐metal carbodiimide Cr2(NCN)3, which shows excellent performance as a negative electrode material in Li‐ion batteries, is conducted combining complementary operando analyses and state‐of‐the‐art density functional theory (DFT) calculations. As predicted by DFT, and evidenced by operando X‐ray diffraction and Cr K‐edge absorption spectroscopy, a two‐step reaction pathway involving two redox couples (Cr3+/Cr2+ and Cr2+/Cr0) and a concomitant formation of Cr metal nanoparticles is apparent, thus indicating that the conversion reaction of this carbodiimide upon lithiation occurs only after a preliminary intercalation step involving two Li per unit formula. This mechanism, evidenced for the first time in transition‐metal carbodiimides, is likely behind its outstanding electrochemical performance as Cr2(NCN)3 can maintain more than 600 mAh g−1 for 900 cycles at a high rate of 2 C.
中文翻译:

锂离子电池Cr2(NCN)3负极的可逆高容量及反应机理
结合互补操作分析和状态分析,对锂与三价过渡金属碳二亚胺Cr 2(NCN)3之间的电化学反应机理进行了详细研究,显示出作为锂离子电池负极材料的出色性能。最新的密度泛函理论(DFT)计算。如DFT所预测,并通过操作X射线衍射和Cr K边缘吸收光谱法证明,这是一个涉及两个氧化还原对(Cr 3+ / Cr 2+和Cr 2+ / Cr 0的两步反应路径))和Cr金属纳米颗粒的伴随形成是显而易见的,因此表明这种碳二亚胺在锂化时的转化反应仅在涉及每单位分子式两个Li的初步嵌入步骤之后发生。该机理首次在过渡金属碳二亚胺中得到证明,可能是因为其出色的电化学性能,因为Cr 2(NCN)3在2 C的高速率下可在900个循环中保持600 mAh g -1以上。
更新日期:2020-01-29
中文翻译:

锂离子电池Cr2(NCN)3负极的可逆高容量及反应机理
结合互补操作分析和状态分析,对锂与三价过渡金属碳二亚胺Cr 2(NCN)3之间的电化学反应机理进行了详细研究,显示出作为锂离子电池负极材料的出色性能。最新的密度泛函理论(DFT)计算。如DFT所预测,并通过操作X射线衍射和Cr K边缘吸收光谱法证明,这是一个涉及两个氧化还原对(Cr 3+ / Cr 2+和Cr 2+ / Cr 0的两步反应路径))和Cr金属纳米颗粒的伴随形成是显而易见的,因此表明这种碳二亚胺在锂化时的转化反应仅在涉及每单位分子式两个Li的初步嵌入步骤之后发生。该机理首次在过渡金属碳二亚胺中得到证明,可能是因为其出色的电化学性能,因为Cr 2(NCN)3在2 C的高速率下可在900个循环中保持600 mAh g -1以上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号