当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zinc Acetate Catalyzed Enantioselective Reductive Aldol Reaction of Ketones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-24 , DOI: 10.1002/adsc.201901457 Izabela Węglarz 1 , Marcin Szewczyk 2 , Jacek Mlynarski 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-24 , DOI: 10.1002/adsc.201901457 Izabela Węglarz 1 , Marcin Szewczyk 2 , Jacek Mlynarski 1
Affiliation
|
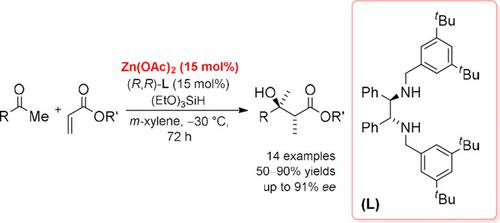
A highly enantioselective method for the synthesis of β‐hydroxy esters via reductive aldol reaction of acrylates with aryl and heteroaromatic ketones is described. In situ generated catalyst composed of zinc acetate and chiral diamine afforded enantioenriched tertiary alcohols in high yields and with excellent enantioselectivity (up to 91% ee). This is also the first successful application of the zinc hydride reagent in stereoselective reductive aldol reactions of ketones.
中文翻译:

醋酸锌催化酮的对映选择性还原醛醇缩合反应
描述了一种通过丙烯酸酯与芳基和杂芳族酮的还原性醛醇缩合反应合成β-羟基酯的高对映选择性的方法。原位生成的由乙酸锌和手性二胺组成的催化剂可以高收率和优异的对映选择性(最高91%ee)提供对映体富集的叔醇 。这也是氢化锌试剂在酮的立体选择性还原羟醛反应中的首次成功应用。
更新日期:2020-01-24
中文翻译:

醋酸锌催化酮的对映选择性还原醛醇缩合反应
描述了一种通过丙烯酸酯与芳基和杂芳族酮的还原性醛醇缩合反应合成β-羟基酯的高对映选择性的方法。原位生成的由乙酸锌和手性二胺组成的催化剂可以高收率和优异的对映选择性(最高91%ee)提供对映体富集的叔醇 。这也是氢化锌试剂在酮的立体选择性还原羟醛反应中的首次成功应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号