当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Observation of Four-Fold Boron-Metal Bonds in RhB(BO-) and RhB.
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.jpclett.9b03484 Ling Fung Cheung 1 , Teng-Teng Chen 1 , G Stephen Kocheril 1 , Wei-Jia Chen 1 , Joseph Czekner 1 , Lai-Sheng Wang 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.jpclett.9b03484 Ling Fung Cheung 1 , Teng-Teng Chen 1 , G Stephen Kocheril 1 , Wei-Jia Chen 1 , Joseph Czekner 1 , Lai-Sheng Wang 1
Affiliation
|
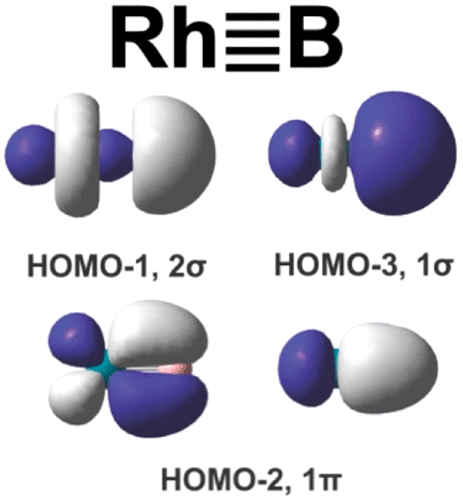
The maximum bond order between two main-group atoms was known to be three. However, it has been suggested recently that there is quadruple bonding in C2 and analogous eight-valence electron species. While the quadruple bond in C2 has aroused some debates, an interesting question is: are main-group elements capable of forming quadruple bonds? Here we use photoelectron spectroscopy and computational chemistry to probe the electronic structure and chemical bonding in RhB2O- and RhB- and show that the boron atom engages in quadruple bonding with rhodium in RhB(BO)- and neutral RhB. The quadruple bonds consist of two π-bonds formed between the Rh 4dxz/4dyz and B 2px/2py orbitals and two σ-bonds between the Rh 4dz2 and B 2s/2pz orbitals. To confirm the quadruple bond in RhB, we also investigate the linear Rh≡B-H+ species and find a triple bond between Rh and B, which has a longer bond length, lower stretching frequency, and smaller bond dissociation energy in comparison with that of the Rh≣B quadruple bond in RhB.
中文翻译:

RhB(BO-)和RhB中四重硼金属键的观察。
已知两个主原子之间的最大键序为3。然而,最近已经提出在C 2和类似的八价电子物种中存在四重键合。尽管C2中的四重键引起了一些争论,但一个有趣的问题是:主族元素是否能够形成四重键?在这里,我们使用光电子能谱和计算化学来探测RhB2O-和RhB-中的电子结构和化学键,并显示硼原子与RhB(BO)-和中性RhB中的铑进行四重键合。四重键由在Rh 4dxz / 4dyz和B 2px / 2py轨道之间形成的两个π键以及在Rh 4dz2和B 2s / 2pz轨道之间的两个σ键组成。为了确认RhB中的四键,
更新日期:2020-01-13
中文翻译:

RhB(BO-)和RhB中四重硼金属键的观察。
已知两个主原子之间的最大键序为3。然而,最近已经提出在C 2和类似的八价电子物种中存在四重键合。尽管C2中的四重键引起了一些争论,但一个有趣的问题是:主族元素是否能够形成四重键?在这里,我们使用光电子能谱和计算化学来探测RhB2O-和RhB-中的电子结构和化学键,并显示硼原子与RhB(BO)-和中性RhB中的铑进行四重键合。四重键由在Rh 4dxz / 4dyz和B 2px / 2py轨道之间形成的两个π键以及在Rh 4dz2和B 2s / 2pz轨道之间的两个σ键组成。为了确认RhB中的四键,











































 京公网安备 11010802027423号
京公网安备 11010802027423号