当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and antioxidant assay of new nicotinonitrile analogues clubbed thiazole, pyrazole and/or pyridine ring systems
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-01-09 , DOI: 10.1002/jhet.3820 Hana M.A. Abumelha 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-01-09 , DOI: 10.1002/jhet.3820 Hana M.A. Abumelha 1
Affiliation
|
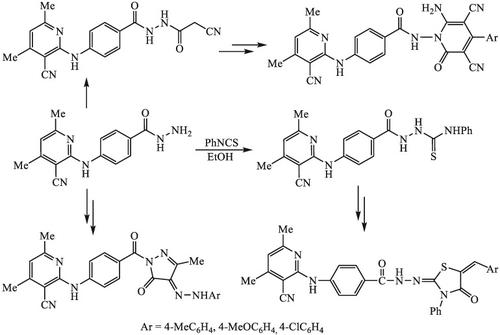
A series of novel nicotinonitrile derivatives were synthesized by hybridization with thiazole, pyrazole, and pyridine ring systems using 4‐aminobenzohydrazide as link‐bridge. The synthetic strategy of nicotinonitrile‐thiazole analogues involves cyclization of the precursor N‐phenyl thiosemicarbazide derivative 4 with chloroacetic acid and phenacyl bromide. The reaction of hydrazide 3 with acetylacetone and/or ethyl acetoacetate was applied as a synthetic route for accessing 2‐((4‐(pyrazole‐1‐carbonyl)phenyl)amino)‐nicotinonitrile derivatives 9–10. The 2‐((4‐(4‐thiazolylidene‐pyrazole‐1‐carbonyl)‐phenyl)amino)nicotinonitriles 14–15 were obtained via a nucleophilic addition of pyrazolone 10 to phenyl isothiocyanate followed by cyclization with chloroacetone, phenacyl chloride, and/or ethyl bromoacetate. The 6‐amino‐4‐aryl‐3,5‐dicyano‐2‐oxo‐1‐(4‐substitutedbenzamido)‐pyridines 19 were synthesized by Knoevenagel condensation N′‐(2‐cyanoacetyl)‐benzohydrazide derivative 16 with substituted benzaldehydes followed by heating with malononitrile. All synthesized products were evaluated for their antioxidant potentialities using of 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid) (ABTS) radical cation delcolorization assay. The nicotinonitrile‐thiazole hybrid 6b was found the most promising antioxidant agent with inhibition activity 86.27%.
中文翻译:

新的烟腈类似物棒状噻唑,吡唑和/或吡啶环系统的合成和抗氧化剂测定
通过使用4-氨基苯甲酰肼作为连接桥与噻唑,吡唑和吡啶环系统杂交,合成了一系列新的烟腈衍生物。烟腈-噻唑类似物的合成策略涉及将前体N-苯基硫代氨基脲衍生物4与氯乙酸和苯甲酰溴环化。酰肼3与乙酰丙酮和/或乙酰乙酸乙酯的反应被用作合成2-((4-(吡唑-1-羰基)苯基)氨基)-烟腈衍生物9-10的合成途径。通过吡咯烷酮10的亲核加成获得2-((4-(4-噻唑基-吡唑-1-羰基)-苯基)氨基)烟腈14-15生成异硫氰酸苯酯,然后用氯丙酮,苯甲酰氯和/或溴代乙酸乙酯环化。6-氨基-4-芳基-3,5-二氰基-2-氧代-1-(4-取代的苯甲酰胺基)-吡啶19是通过Knoevenagel缩合N' -(2-氰基乙酰基)-苯并酰肼衍生物16与取代的苯甲醛合成的用丙二腈加热。使用2,2'-叠氮基-双(3-乙基苯并噻唑啉-6-磺酸)(ABTS)自由基阳离子脱色法评估所有合成产物的抗氧化能力。烟腈-噻唑杂化物6b被发现是最有前途的抗氧化剂,其抑制活性为86.27%。
更新日期:2020-01-09
中文翻译:

新的烟腈类似物棒状噻唑,吡唑和/或吡啶环系统的合成和抗氧化剂测定
通过使用4-氨基苯甲酰肼作为连接桥与噻唑,吡唑和吡啶环系统杂交,合成了一系列新的烟腈衍生物。烟腈-噻唑类似物的合成策略涉及将前体N-苯基硫代氨基脲衍生物4与氯乙酸和苯甲酰溴环化。酰肼3与乙酰丙酮和/或乙酰乙酸乙酯的反应被用作合成2-((4-(吡唑-1-羰基)苯基)氨基)-烟腈衍生物9-10的合成途径。通过吡咯烷酮10的亲核加成获得2-((4-(4-噻唑基-吡唑-1-羰基)-苯基)氨基)烟腈14-15生成异硫氰酸苯酯,然后用氯丙酮,苯甲酰氯和/或溴代乙酸乙酯环化。6-氨基-4-芳基-3,5-二氰基-2-氧代-1-(4-取代的苯甲酰胺基)-吡啶19是通过Knoevenagel缩合N' -(2-氰基乙酰基)-苯并酰肼衍生物16与取代的苯甲醛合成的用丙二腈加热。使用2,2'-叠氮基-双(3-乙基苯并噻唑啉-6-磺酸)(ABTS)自由基阳离子脱色法评估所有合成产物的抗氧化能力。烟腈-噻唑杂化物6b被发现是最有前途的抗氧化剂,其抑制活性为86.27%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号