当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Cyclic Amidines from Quinolines by a Borane-Catalyzed Dearomatization Strategy.
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.orglett.9b04275 Vinh Do Cao 1 , So Hwa Mun 1 , Seo Ha Kim 1 , Gyeong Un Kim 1 , Hyung Guk Kim 1 , Seewon Joung 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.orglett.9b04275 Vinh Do Cao 1 , So Hwa Mun 1 , Seo Ha Kim 1 , Gyeong Un Kim 1 , Hyung Guk Kim 1 , Seewon Joung 1
Affiliation
|
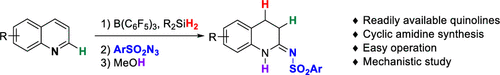
Described herein is the development of a new synthetic route to cyclic amidines from quinolines. The borane-catalyzed 1,4-hydrosilylation of quinoline was utilized for the dearomatization of the quinolines. The dearomatized enamine intermediate was subsequently reacted with a broad range of organic azides to produce the corresponding cyclic amidines (3,4-dihydroquinolinimines) via a [3 + 2] cycloaddition pathway. Preliminary mechanistic studies suggested that the hydride shift was involved during the cycloaddition.
中文翻译:

通过硼烷催化的脱芳香化策略由喹啉合成环Am。
本文描述了从喹啉向环am的新合成路线的开发。硼烷催化的喹啉的1,4-氢化硅烷化被用于喹啉的脱芳香化。随后将脱芳香化的烯胺中间体与各种有机叠氮化物反应,以通过[3 + 2]环加成途径生成相应的环状am(3,4-二氢喹啉亚胺)。初步的机理研究表明,在环加成反应中涉及到氢化物的转变。
更新日期:2020-01-08
中文翻译:

通过硼烷催化的脱芳香化策略由喹啉合成环Am。
本文描述了从喹啉向环am的新合成路线的开发。硼烷催化的喹啉的1,4-氢化硅烷化被用于喹啉的脱芳香化。随后将脱芳香化的烯胺中间体与各种有机叠氮化物反应,以通过[3 + 2]环加成途径生成相应的环状am(3,4-二氢喹啉亚胺)。初步的机理研究表明,在环加成反应中涉及到氢化物的转变。











































 京公网安备 11010802027423号
京公网安备 11010802027423号