当前位置:
X-MOL 学术
›
Blood Cancer J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new regulatory mechanism of protein phosphatase 2A activity via SET in acute myeloid leukemia.
Blood Cancer Journal ( IF 12.9 ) Pub Date : 2020-01-08 , DOI: 10.1038/s41408-019-0270-0 Elena Arriazu 1, 2 , Carmen Vicente 1, 3 , Raffaella Pippa 1, 4 , Irene Peris 1, 3 , Elena Martínez-Balsalobre 5 , Patricia García-Ramírez 6 , Nerea Marcotegui 1 , Ana Igea 7 , Diego Alignani 1, 2 , José Rifón 1, 2, 8 , María C Mateos 6, 8 , María L Cayuela 5 , Angel R Nebreda 7, 9 , María D Odero 1, 2, 3, 8
Blood Cancer Journal ( IF 12.9 ) Pub Date : 2020-01-08 , DOI: 10.1038/s41408-019-0270-0 Elena Arriazu 1, 2 , Carmen Vicente 1, 3 , Raffaella Pippa 1, 4 , Irene Peris 1, 3 , Elena Martínez-Balsalobre 5 , Patricia García-Ramírez 6 , Nerea Marcotegui 1 , Ana Igea 7 , Diego Alignani 1, 2 , José Rifón 1, 2, 8 , María C Mateos 6, 8 , María L Cayuela 5 , Angel R Nebreda 7, 9 , María D Odero 1, 2, 3, 8
Affiliation
|
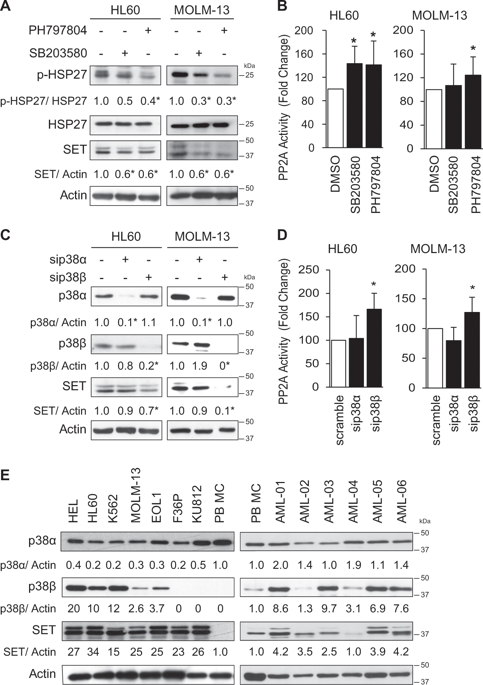
Acute myeloid leukemia (AML) is an aggressive hematologic malignancy. Although novel emerging drugs are available, the overall prognosis remains poor and new therapeutic approaches are required. PP2A phosphatase is a key regulator of cell homeostasis and is recurrently inactivated in AML. The anticancer activity of several PP2A-activating drugs (e.g., FTY720) depends on their interaction with the SET oncoprotein, an endogenous PP2A inhibitor that is overexpressed in 30% of AML cases. Elucidation of SET regulatory mechanisms may therefore provide novel targeted therapies for SET-overexpressing AMLs. Here, we show that upregulation of protein kinase p38β is a common event in AML. We provide evidence that p38β potentiates SET-mediated PP2A inactivation by two mechanisms: facilitating SET cytoplasmic translocation through CK2 phosphorylation, and directly binding to and stabilizing the SET protein. We demonstrate the importance of this new regulatory mechanism in primary AML cells from patients and in zebrafish xenograft models. Accordingly, combination of the CK2 inhibitor CX-4945, which retains SET in the nucleus, and FTY720, which disrupts the SET-PP2A binding in the cytoplasm, significantly reduces the viability and migration of AML cells. In conclusion, we show that the p38β/CK2/SET axis represents a new potential therapeutic pathway in AML patients with SET-dependent PP2A inactivation.
中文翻译:

通过SET在急性髓细胞性白血病中蛋白磷酸酶2A活性的新调节机制。
急性髓细胞性白血病(AML)是一种侵袭性血液恶性肿瘤。尽管可以使用新型新兴药物,但总体预后仍然很差,需要新的治疗方法。PP2A磷酸酶是细胞稳态的关键调节剂,在AML中经常失活。几种PP2A激活药物(例如FTY720)的抗癌活性取决于它们与SET癌蛋白的相互作用,SET癌蛋白是一种内源性PP2A抑制剂,在30%的AML病例中过表达。因此,SET调节机制的阐明可为过表达SET的AML提供新颖的靶向疗法。在这里,我们显示蛋白激酶p38β的上调是AML中的常见事件。我们提供的证据表明,p38β通过以下两种机制增强SET介导的PP2A失活:通过CK2磷酸化促进SET细胞质易位,并直接结合并稳定SET蛋白。我们证明了这种新的调节机制在患者原代AML细胞和斑马鱼异种移植模型中的重要性。因此,将CK保留在细胞核中的CK2抑制剂CX-4945和破坏细胞质中SET-PP2A结合的FTY720的组合显着降低了AML细胞的活力和迁移。总之,我们显示p38β/ CK2 / SET轴代表SET依赖性PP2A失活的AML患者的新的潜在治疗途径。这破坏了SET-PP2A在细胞质中的结合,显着降低了AML细胞的活力和迁移。总之,我们显示p38β/ CK2 / SET轴代表SET依赖性PP2A失活的AML患者的新的潜在治疗途径。这破坏了SET-PP2A在细胞质中的结合,显着降低了AML细胞的活力和迁移。总之,我们显示p38β/ CK2 / SET轴代表SET依赖性PP2A失活的AML患者的新的潜在治疗途径。
更新日期:2020-01-08
中文翻译:

通过SET在急性髓细胞性白血病中蛋白磷酸酶2A活性的新调节机制。
急性髓细胞性白血病(AML)是一种侵袭性血液恶性肿瘤。尽管可以使用新型新兴药物,但总体预后仍然很差,需要新的治疗方法。PP2A磷酸酶是细胞稳态的关键调节剂,在AML中经常失活。几种PP2A激活药物(例如FTY720)的抗癌活性取决于它们与SET癌蛋白的相互作用,SET癌蛋白是一种内源性PP2A抑制剂,在30%的AML病例中过表达。因此,SET调节机制的阐明可为过表达SET的AML提供新颖的靶向疗法。在这里,我们显示蛋白激酶p38β的上调是AML中的常见事件。我们提供的证据表明,p38β通过以下两种机制增强SET介导的PP2A失活:通过CK2磷酸化促进SET细胞质易位,并直接结合并稳定SET蛋白。我们证明了这种新的调节机制在患者原代AML细胞和斑马鱼异种移植模型中的重要性。因此,将CK保留在细胞核中的CK2抑制剂CX-4945和破坏细胞质中SET-PP2A结合的FTY720的组合显着降低了AML细胞的活力和迁移。总之,我们显示p38β/ CK2 / SET轴代表SET依赖性PP2A失活的AML患者的新的潜在治疗途径。这破坏了SET-PP2A在细胞质中的结合,显着降低了AML细胞的活力和迁移。总之,我们显示p38β/ CK2 / SET轴代表SET依赖性PP2A失活的AML患者的新的潜在治疗途径。这破坏了SET-PP2A在细胞质中的结合,显着降低了AML细胞的活力和迁移。总之,我们显示p38β/ CK2 / SET轴代表SET依赖性PP2A失活的AML患者的新的潜在治疗途径。










































 京公网安备 11010802027423号
京公网安备 11010802027423号