当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Geometry of Hydrogen Bonds in Liquid Ethanol Probed by Proton NMR Experiments.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.jpcb.9b10184 Ritu Ghanghas 1 , Aman Jindal 1 , Sukumaran Vasudevan 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.jpcb.9b10184 Ritu Ghanghas 1 , Aman Jindal 1 , Sukumaran Vasudevan 1
Affiliation
|
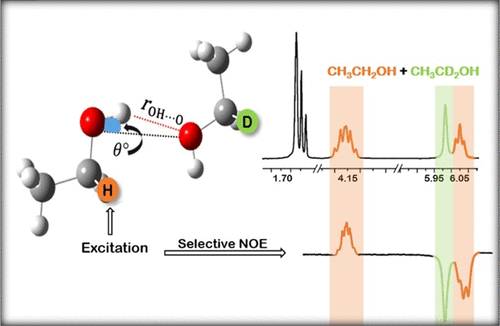
Quantitative information of hydrogen bonding is crucial to our understanding of the structure and properties of associated liquids. Here, we outline a simple procedure to establish the geometry of hydrogen bonds in liquid ethanol using proton nuclear magnetic resonance (NMR) spectroscopy. We do so by exploiting differences in proton chemical shift values, that originate from the secondary isotope effect, to distinguish the methyl and hydroxyl group protons of CH3CH2OH from those of the deuterated CH3CD2OH in the 1H NMR spectra of mixtures of the two. This has allowed us to measure the ratios of the inter- to intramolecular distances between methyl to hydroxyl and methylene to hydroxyl protons using one-dimensional (1D) transient nuclear Overhauser effect NMR measurements as a molecular ruler. We model liquid ethanol by ab initio molecular dynamics simulations and identify all possible pairs of ethanol molecules in the ensemble that satisfied the NMR-determined inter- to intramolecular distance ratio criteria. For these pairs of ethanol molecules, we find the mean value of the hydrogen bonding distance, rOH···O, to be 1.93 Å and the value of the ∠HO···O angle to be 13.3°, thus effectively establishing the geometry of hydrogen bonds in liquid ethanol. An interesting observation that emerges from our study is the linear correlation between hydrogen bond distances and angles in ethanol.
中文翻译:

质子核磁共振实验探测液体乙醇中氢键的几何形状。
氢键的定量信息对于我们对相关液体的结构和性质的理解至关重要。在这里,我们概述了使用质子核磁共振(NMR)光谱建立液体乙醇中氢键几何结构的简单程序。我们通过利用源自次级同位素效应的质子化学位移值的差异,来区分两者混合物的1H NMR谱中CH3CH2OH的甲基和羟基质子与氘代CH3CD2OH的质子。这使我们能够使用一维(1D)瞬态核Overhauser效应NMR测量作为分子尺来测量甲基与羟基之间的分子间距离与分子内分子之间的比率,以及亚甲基与羟基质子之间的距离。我们通过从头算分子动力学模拟对液体乙醇进行建模,并确定满足NMR确定的分子间与分子内距离比标准的整体中所有可能的乙醇分子对。对于这些成对的乙醇分子,我们发现氢键合距离的平均值rOH···O为1.93Å,·HO···O角的值为13.3°,从而有效地建立了几何形状液体乙醇中的氢键 从我们的研究中得出的有趣发现是乙醇中氢键距离和角度之间的线性相关性。我们发现氢键合距离的平均值rOH···O为1.93Å,∠HO··O角的值为13.3°,从而有效地建立了液体乙醇中氢键的几何形状。从我们的研究中得出的有趣发现是乙醇中氢键距离和角度之间的线性相关性。我们发现氢键合距离的平均值rOH···O为1.93Å,∠HO··O角的值为13.3°,从而有效地建立了液体乙醇中氢键的几何形状。从我们的研究中得出的有趣发现是乙醇中氢键距离和角度之间的线性相关性。
更新日期:2020-01-17
中文翻译:

质子核磁共振实验探测液体乙醇中氢键的几何形状。
氢键的定量信息对于我们对相关液体的结构和性质的理解至关重要。在这里,我们概述了使用质子核磁共振(NMR)光谱建立液体乙醇中氢键几何结构的简单程序。我们通过利用源自次级同位素效应的质子化学位移值的差异,来区分两者混合物的1H NMR谱中CH3CH2OH的甲基和羟基质子与氘代CH3CD2OH的质子。这使我们能够使用一维(1D)瞬态核Overhauser效应NMR测量作为分子尺来测量甲基与羟基之间的分子间距离与分子内分子之间的比率,以及亚甲基与羟基质子之间的距离。我们通过从头算分子动力学模拟对液体乙醇进行建模,并确定满足NMR确定的分子间与分子内距离比标准的整体中所有可能的乙醇分子对。对于这些成对的乙醇分子,我们发现氢键合距离的平均值rOH···O为1.93Å,·HO···O角的值为13.3°,从而有效地建立了几何形状液体乙醇中的氢键 从我们的研究中得出的有趣发现是乙醇中氢键距离和角度之间的线性相关性。我们发现氢键合距离的平均值rOH···O为1.93Å,∠HO··O角的值为13.3°,从而有效地建立了液体乙醇中氢键的几何形状。从我们的研究中得出的有趣发现是乙醇中氢键距离和角度之间的线性相关性。我们发现氢键合距离的平均值rOH···O为1.93Å,∠HO··O角的值为13.3°,从而有效地建立了液体乙醇中氢键的几何形状。从我们的研究中得出的有趣发现是乙醇中氢键距离和角度之间的线性相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号