当前位置:
X-MOL 学术
›
Mater. Sci. Semicond. Proc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Co2+ content on supercapacitance properties of hydrothermally synthesized Ni1-xCoxFe2O4 nanoparticles
Materials Science in Semiconductor Processing ( IF 4.2 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.mssp.2019.104902 Samira Sharifi , Ahmad Yazdani , Kourosh Rahimi
Materials Science in Semiconductor Processing ( IF 4.2 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.mssp.2019.104902 Samira Sharifi , Ahmad Yazdani , Kourosh Rahimi
|
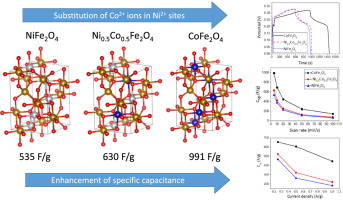
Abstract Rich physical properties of ferrite nanoparticles, which exhibit a spinel structure, are sensitive to the distribution of cations in tetrahedral and octahedral sites as well as the crystal field effect, both of which can modify the electronic structure. Here, using a hydrothermal method, we incorporated Co+2 ions into nickel ferrite (NiFe2O4) nanoparticles to synthesize Ni1-xCoxFe2O4 (x = 0.0, 0.5, 1) nanoparticles and studied how their structural, electronic, and electrochemical properties are influenced by the Co+2 content. We found that the employed surfactant agent (here, CTAB) is critically important and affects the size and the specific surface area of the nanoparticles. X-ray diffractometry indicated that the obtained nanoparticles have an inverse spinel structure and field emission scanning electron microscopy showed that the nanoparticles' size is in the range 15–20 nm. Fourier-transform infrared spectroscopy and energy dispersive X-ray spectroscopy confirmed the chemical compositions of the samples. UV–visible spectrophotometry indicated that the optical bandgap of Ni1-xCoxFe2O4 decreases from 2.2 to 1.95 eV as the Co+2 content increases. Electrochemical properties of the samples were studied using cycling voltammetry, galvanostatic charge-discharge, and electrochemical impedance spectroscopy techniques. The specific capacitances achieved by the supercapacitors constructed from the prepared NiFe2O4, Ni0.5Co0.5Fe2O4, and CoFe2O4 nanoparticles were calculated from cycling voltammetry curves at the scan rate of 5 mV/s as 534.5, 630, and 991 F/g, respectively, and from galvanostatic charge-discharge curves at the current density of 0.25 A/g as 467.43, 522.80 and 655.60 F/g, respectively. We discussed that the increase in the specific capacitances as the Co+2 content increases is because the Co+2 content increases the available ways for the electrode to react with the electrolyte. Finally, we performed a density functional theory study on the structures to provide more insight into the increased specific capacitance.
中文翻译:

Co2+含量对水热合成Ni1-xCoxFe2O4纳米颗粒超级电容性能的影响
摘要 铁氧体纳米颗粒具有尖晶石结构,其丰富的物理性质对阳离子在四面体和八面体位点的分布以及晶体场效应敏感,两者都可以改变电子结构。在这里,我们使用水热法将 Co+2 离子掺入铁氧体镍 (NiFe2O4) 纳米粒子中以合成 Ni1-xCoxFe2O4 (x = 0.0, 0.5, 1) 纳米粒子,并研究了它们的结构、电子和电化学性质如何受Co+2 含量。我们发现所采用的表面活性剂(此处为 CTAB)至关重要,并且会影响纳米颗粒的尺寸和比表面积。X 射线衍射表明获得的纳米颗粒具有反尖晶石结构,场发射扫描电子显微镜显示纳米颗粒的尺寸在 15-20 nm 范围内。傅里叶变换红外光谱和能量色散 X 射线光谱证实了样品的化学成分。紫外可见分光光度法表明,随着 Co+2 含量的增加,Ni1-xCoxFe2O4 的光学带隙从 2.2 eV 降低到 1.95 eV。使用循环伏安法、恒电流充放电和电化学阻抗谱技术研究样品的电化学性质。由制备的 NiFe2O4、Ni0.5Co0.5Fe2O4、CoFe2O4 和 CoFe2O4 纳米粒子根据扫描速率为 5mV/s 的循环伏安曲线计算分别为 534.5、630 和 991 F/g,从电流密度为 0.25 A/g 的恒电流充放电曲线计算为 467.43,分别为 522.80 和 655.60 F/g。我们讨论过,随着 Co+2 含量的增加,比电容的增加是因为 Co+2 含量增加了电极与电解质反应的可用方式。最后,我们对结构进行了密度泛函理论研究,以更深入地了解增加的比电容。我们讨论过,随着 Co+2 含量的增加,比电容的增加是因为 Co+2 含量增加了电极与电解质反应的可用方式。最后,我们对结构进行了密度泛函理论研究,以更深入地了解增加的比电容。我们讨论过,随着 Co+2 含量的增加,比电容的增加是因为 Co+2 含量增加了电极与电解质反应的可用方式。最后,我们对结构进行了密度泛函理论研究,以更深入地了解增加的比电容。
更新日期:2020-03-01
中文翻译:

Co2+含量对水热合成Ni1-xCoxFe2O4纳米颗粒超级电容性能的影响
摘要 铁氧体纳米颗粒具有尖晶石结构,其丰富的物理性质对阳离子在四面体和八面体位点的分布以及晶体场效应敏感,两者都可以改变电子结构。在这里,我们使用水热法将 Co+2 离子掺入铁氧体镍 (NiFe2O4) 纳米粒子中以合成 Ni1-xCoxFe2O4 (x = 0.0, 0.5, 1) 纳米粒子,并研究了它们的结构、电子和电化学性质如何受Co+2 含量。我们发现所采用的表面活性剂(此处为 CTAB)至关重要,并且会影响纳米颗粒的尺寸和比表面积。X 射线衍射表明获得的纳米颗粒具有反尖晶石结构,场发射扫描电子显微镜显示纳米颗粒的尺寸在 15-20 nm 范围内。傅里叶变换红外光谱和能量色散 X 射线光谱证实了样品的化学成分。紫外可见分光光度法表明,随着 Co+2 含量的增加,Ni1-xCoxFe2O4 的光学带隙从 2.2 eV 降低到 1.95 eV。使用循环伏安法、恒电流充放电和电化学阻抗谱技术研究样品的电化学性质。由制备的 NiFe2O4、Ni0.5Co0.5Fe2O4、CoFe2O4 和 CoFe2O4 纳米粒子根据扫描速率为 5mV/s 的循环伏安曲线计算分别为 534.5、630 和 991 F/g,从电流密度为 0.25 A/g 的恒电流充放电曲线计算为 467.43,分别为 522.80 和 655.60 F/g。我们讨论过,随着 Co+2 含量的增加,比电容的增加是因为 Co+2 含量增加了电极与电解质反应的可用方式。最后,我们对结构进行了密度泛函理论研究,以更深入地了解增加的比电容。我们讨论过,随着 Co+2 含量的增加,比电容的增加是因为 Co+2 含量增加了电极与电解质反应的可用方式。最后,我们对结构进行了密度泛函理论研究,以更深入地了解增加的比电容。我们讨论过,随着 Co+2 含量的增加,比电容的增加是因为 Co+2 含量增加了电极与电解质反应的可用方式。最后,我们对结构进行了密度泛函理论研究,以更深入地了解增加的比电容。











































 京公网安备 11010802027423号
京公网安备 11010802027423号