当前位置:
X-MOL 学术
›
Mol. Psychiatry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Increased surface P2X4 receptor regulates anxiety and memory in P2X4 internalization-defective knock-in mice.
Molecular Psychiatry ( IF 9.6 ) Pub Date : 2020-01-08 , DOI: 10.1038/s41380-019-0641-8 Eléonore Bertin 1, 2 , Thomas Deluc 1, 2, 3 , Kjara S Pilch 1, 2 , Audrey Martinez 1, 2 , Johan-Till Pougnet 1, 2 , Evelyne Doudnikoff 1, 2 , Anne-Emilie Allain 4, 5 , Philine Bergmann 6 , Marion Russeau 7 , Estelle Toulmé 1, 2 , Erwan Bezard 1, 2 , Friedrich Koch-Nolte 6 , Philippe Séguéla 3 , Sabine Lévi 7 , Bruno Bontempi 1, 2 , François Georges 1, 2 , Sandrine S Bertrand 4, 5 , Olivier Nicole 1, 2 , Eric Boué-Grabot 1, 2
Molecular Psychiatry ( IF 9.6 ) Pub Date : 2020-01-08 , DOI: 10.1038/s41380-019-0641-8 Eléonore Bertin 1, 2 , Thomas Deluc 1, 2, 3 , Kjara S Pilch 1, 2 , Audrey Martinez 1, 2 , Johan-Till Pougnet 1, 2 , Evelyne Doudnikoff 1, 2 , Anne-Emilie Allain 4, 5 , Philine Bergmann 6 , Marion Russeau 7 , Estelle Toulmé 1, 2 , Erwan Bezard 1, 2 , Friedrich Koch-Nolte 6 , Philippe Séguéla 3 , Sabine Lévi 7 , Bruno Bontempi 1, 2 , François Georges 1, 2 , Sandrine S Bertrand 4, 5 , Olivier Nicole 1, 2 , Eric Boué-Grabot 1, 2
Affiliation
|
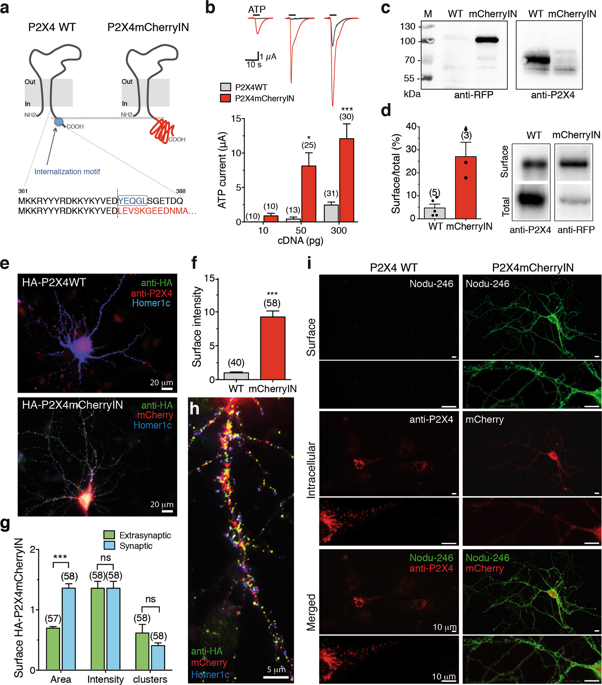
ATP signaling and surface P2X4 receptors are upregulated selectively in neurons and/or glia in various CNS disorders including anxiety, chronic pain, epilepsy, ischemia, and neurodegenerative diseases. However, the cell-specific functions of P2X4 in pathological contexts remain elusive. To elucidate P2X4 functions, we created a conditional transgenic knock-in P2X4 mouse line (Floxed P2X4mCherryIN) allowing the Cre activity-dependent genetic swapping of the internalization motif of P2X4 by the fluorescent mCherry protein to prevent constitutive endocytosis of P2X4. By combining molecular, cellular, electrophysiological, and behavioral approaches, we characterized two distinct knock-in mouse lines expressing noninternalized P2X4mCherryIN either exclusively in excitatory forebrain neurons or in all cells natively expressing P2X4. The genetic substitution of wild-type P2X4 by noninternalized P2X4mCherryIN in both knock-in mouse models did not alter the sparse distribution and subcellular localization of P2X4 but increased the number of P2X4 receptors at the surface of the targeted cells mimicking the pathological increased surface P2X4 state. Increased surface P2X4 density in the hippocampus of knock-in mice altered LTP and LTD plasticity phenomena at CA1 synapses without affecting basal excitatory transmission. Moreover, these cellular events translated into anxiolytic effects and deficits in spatial memory. Our results show that increased surface density of neuronal P2X4 contributes to synaptic deficits and alterations in anxiety and memory functions consistent with the implication of P2X4 in neuropsychiatric and neurodegenerative disorders. Furthermore, these conditional P2X4mCherryIN knock-in mice will allow exploring the cell-specific roles of P2X4 in various physiological and pathological contexts.
中文翻译:

增加的表面 P2X4 受体调节 P2X4 内化缺陷敲入小鼠的焦虑和记忆。
ATP 信号传导和表面 P2X4 受体在各种 CNS 疾病(包括焦虑、慢性疼痛、癫痫、缺血和神经退行性疾病)中的神经元和/或神经胶质细胞中选择性上调。然而,P2X4 在病理情况下的细胞特异性功能仍然难以捉摸。为了阐明 P2X4 的功能,我们创建了一个条件性转基因敲入 P2X4 小鼠系 (Floxed P2X4mCherryIN),允许荧光 mCherry 蛋白对 P2X4 的内化基序进行 Cre 活性依赖性遗传交换,以防止 P2X4 的组成性内吞作用。通过结合分子、细胞、电生理学和行为学方法,我们表征了两种不同的敲入小鼠系,它们表达非内化 P2X4mCherryIN,它们要么仅在兴奋性前脑神经元中表达,要么在所有天然表达 P2X4 的细胞中表达。在两种敲入小鼠模型中,非内化 P2X4mCherryIN 对野生型 P2X4 的遗传取代并未改变 P2X4 的稀疏分布和亚细胞定位,但增加了靶细胞表面 P2X4 受体的数量,模拟病理性增加的表面 P2X4 状态. 敲入小鼠海马体表面 P2X4 密度的增加改变了 CA1 突触的 LTP 和 LTD 可塑性现象,而不影响基础兴奋性传递。此外,这些细胞事件转化为抗焦虑作用和空间记忆缺陷。我们的研究结果表明,神经元 P2X4 表面密度的增加会导致突触缺陷以及焦虑和记忆功能的改变,这与 P2X4 在神经精神和神经退行性疾病中的意义一致。此外,
更新日期:2020-01-08
中文翻译:

增加的表面 P2X4 受体调节 P2X4 内化缺陷敲入小鼠的焦虑和记忆。
ATP 信号传导和表面 P2X4 受体在各种 CNS 疾病(包括焦虑、慢性疼痛、癫痫、缺血和神经退行性疾病)中的神经元和/或神经胶质细胞中选择性上调。然而,P2X4 在病理情况下的细胞特异性功能仍然难以捉摸。为了阐明 P2X4 的功能,我们创建了一个条件性转基因敲入 P2X4 小鼠系 (Floxed P2X4mCherryIN),允许荧光 mCherry 蛋白对 P2X4 的内化基序进行 Cre 活性依赖性遗传交换,以防止 P2X4 的组成性内吞作用。通过结合分子、细胞、电生理学和行为学方法,我们表征了两种不同的敲入小鼠系,它们表达非内化 P2X4mCherryIN,它们要么仅在兴奋性前脑神经元中表达,要么在所有天然表达 P2X4 的细胞中表达。在两种敲入小鼠模型中,非内化 P2X4mCherryIN 对野生型 P2X4 的遗传取代并未改变 P2X4 的稀疏分布和亚细胞定位,但增加了靶细胞表面 P2X4 受体的数量,模拟病理性增加的表面 P2X4 状态. 敲入小鼠海马体表面 P2X4 密度的增加改变了 CA1 突触的 LTP 和 LTD 可塑性现象,而不影响基础兴奋性传递。此外,这些细胞事件转化为抗焦虑作用和空间记忆缺陷。我们的研究结果表明,神经元 P2X4 表面密度的增加会导致突触缺陷以及焦虑和记忆功能的改变,这与 P2X4 在神经精神和神经退行性疾病中的意义一致。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号