当前位置:
X-MOL 学术
›
JAMA Psychiatry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficacy and Safety of Lumateperone for Treatment of Schizophrenia: A Randomized Clinical Trial.
JAMA Psychiatry ( IF 22.5 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamapsychiatry.2019.4379 Christoph U Correll 1, 2, 3 , Robert E Davis 4 , Michal Weingart 4 , Jelena Saillard 4 , Cedric O'Gorman 4, 5 , John M Kane 1, 2 , Jeffrey A Lieberman 6 , Carol A Tamminga 7 , Sharon Mates 4 , Kimberly E Vanover 4
JAMA Psychiatry ( IF 22.5 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamapsychiatry.2019.4379 Christoph U Correll 1, 2, 3 , Robert E Davis 4 , Michal Weingart 4 , Jelena Saillard 4 , Cedric O'Gorman 4, 5 , John M Kane 1, 2 , Jeffrey A Lieberman 6 , Carol A Tamminga 7 , Sharon Mates 4 , Kimberly E Vanover 4
Affiliation
|
Importance
Individuals living with schizophrenia are affected by cardiometabolic, endocrine, and motor adverse effects of current antipsychotic medications. Lumateperone is a serotonin, dopamine, and glutamate modulator with the potential to treat schizophrenia with few adverse effects.
Objective
To examine the efficacy and safety of lumateperone for the short-term treatment of schizophrenia.
Design, Setting, and Participants
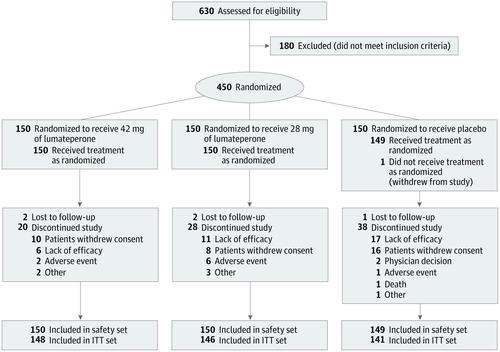
This randomized, double-blind, placebo-controlled, phase 3 clinical trial was conducted from November 13, 2014, to July 20, 2015, with data analyses performed from August 13 to September 15, 2015. Patients with schizophrenia who were aged 18 to 60 years and were experiencing an acute exacerbation of psychosis were enrolled from 12 clinical sites in the United States.
Interventions
Patients were randomized 1:1:1 (150 patients in each arm) to receive lumateperone tosylate, 60 mg; lumateperone tosylate, 40 mg (equivalent to 42 or 28 mg, respectively, of the active moiety lumateperone); or placebo once daily for 4 weeks.
Main Outcomes and Measures
The prespecified primary efficacy end point was mean change from baseline to day 28 in the Positive and Negative Syndrome Scale (PANSS) total score vs placebo. The key secondary efficacy measure was the Clinical Global Impression-Severity of Illness (CGI-S) score. The PANSS subscale scores, social function, safety, and tolerability were also assessed.
Results
The study comprised 450 patients (mean [SD] age, 42.4 [10.2] years; 346 [77.1%] male; mean [SD] baseline PANSS score, 89.8 [10.3]; mean [SD] baseline CGI-S score, 4.8 [0.6]). In the prespecified modified intent-to-treat efficacy analysis (n = 435), 42 mg of lumateperone met the primary and key secondary efficacy objectives, demonstrating a statistically significant improvement vs placebo from baseline to day 28 on the PANSS total score (least-squares mean difference [LSMD], -4.2; 95% CI, -7.8 to -0.6; P = .02; effect size [ES], -0.3) and the CGI-S (LSMD, -0.3; 95% CI, -0.5 to -0.1; P = .003; ES, -0.4). For 28 mg of lumateperone, the LSMD from baseline to day 28 was -2.6 (95% CI, -6.2 to 1.1; P = .16; ES, -0.2) on the PANSS total score and -0.2 (95% CI, -0.5 to 0.0; P = .02; ES, -0.3) on the CGI-S. Both lumateperone doses were well tolerated without clinically significant treatment-emergent motor adverse effects or changes in cardiometabolic or endocrine factors vs placebo.
Conclusions and Relevance
Lumateperone demonstrated efficacy for improving the symptoms of schizophrenia and had a favorable safety profile.
Trial Registration
ClinicalTrials.gov identifier: NCT02282761.
中文翻译:

Lumateperone 治疗精神分裂症的功效和安全性:随机临床试验。
重要性 精神分裂症患者会受到当前抗精神病药物的心脏代谢、内分泌和运动不良反应的影响。 Lumateperone 是一种血清素、多巴胺和谷氨酸调节剂,具有治疗精神分裂症的潜力,且副作用很少。目的探讨鲁美哌隆短期治疗精神分裂症的疗效和安全性。设计、设置和参与者 这项随机、双盲、安慰剂对照的 3 期临床试验于 2014 年 11 月 13 日至 2015 年 7 月 20 日进行,数据分析于 2015 年 8 月 13 日至 9 月 15 日进行。美国 12 个临床中心招募了年龄在 18 至 60 岁且正在经历精神病急性加重的精神分裂症患者。干预措施 患者按照 1:1:1(每组 150 名患者)随机接受甲苯磺酸鲁美特哌隆 60 毫克;甲苯磺酸鲁美特哌隆,40 mg (分别相当于 42 或 28 mg 活性部分鲁美特哌隆);或安慰剂,每天一次,持续 4 周。主要结果和测量 预先指定的主要疗效终点是阳性和阴性综合征量表 (PANSS) 总分与安慰剂相比从基线到第 28 天的平均变化。关键的次要疗效指标是临床总体印象-疾病严重程度(CGI-S)评分。还评估了 PANSS 子量表评分、社会功能、安全性和耐受性。结果 该研究包括 450 名患者(平均 [SD] 年龄,42.4 [10.2] 岁;346 [77.1%] 男性;平均 [SD] 基线 PANSS 评分,89.8 [10.3];平均 [SD] 基线 CGI-S 评分,4.8 [0.6])。 在预先指定的改良意向治疗疗效分析(n = 435)中,42 mg 鲁美特哌隆满足了主要和关键的次要疗效目标,表明从基线到第 28 天,PANSS 总分(至少平方平均差 [LSMD],-4.2;95% CI,-7.8 至 -0.6;P = .02;效应大小 [ES],-0.3)和 CGI-S(LSMD,-0.3;95% CI,- 0.5 至 -0.1;P = .003;ES,-0.4)。对于 28 mg 鲁美哌隆,从基线到第 28 天的 LSMD 为 -2.6(95% CI,-6.2 至 1.1;P = .16;ES,-0.2),PANSS 总分为 -0.2(95% CI,- 0.5 至 0.0;P = .02;ES,-0.3) 在 CGI-S 上。与安慰剂相比,两种鲁美特哌隆剂量均具有良好的耐受性,没有临床上显着的治疗引起的运动不良反应或心脏代谢或内分泌因素的变化。结论和相关性 Lumateperone 可有效改善精神分裂症症状,并具有良好的安全性。试验注册 ClinicalTrials.gov 标识符:NCT02282761。
更新日期:2020-04-01
中文翻译:

Lumateperone 治疗精神分裂症的功效和安全性:随机临床试验。
重要性 精神分裂症患者会受到当前抗精神病药物的心脏代谢、内分泌和运动不良反应的影响。 Lumateperone 是一种血清素、多巴胺和谷氨酸调节剂,具有治疗精神分裂症的潜力,且副作用很少。目的探讨鲁美哌隆短期治疗精神分裂症的疗效和安全性。设计、设置和参与者 这项随机、双盲、安慰剂对照的 3 期临床试验于 2014 年 11 月 13 日至 2015 年 7 月 20 日进行,数据分析于 2015 年 8 月 13 日至 9 月 15 日进行。美国 12 个临床中心招募了年龄在 18 至 60 岁且正在经历精神病急性加重的精神分裂症患者。干预措施 患者按照 1:1:1(每组 150 名患者)随机接受甲苯磺酸鲁美特哌隆 60 毫克;甲苯磺酸鲁美特哌隆,40 mg (分别相当于 42 或 28 mg 活性部分鲁美特哌隆);或安慰剂,每天一次,持续 4 周。主要结果和测量 预先指定的主要疗效终点是阳性和阴性综合征量表 (PANSS) 总分与安慰剂相比从基线到第 28 天的平均变化。关键的次要疗效指标是临床总体印象-疾病严重程度(CGI-S)评分。还评估了 PANSS 子量表评分、社会功能、安全性和耐受性。结果 该研究包括 450 名患者(平均 [SD] 年龄,42.4 [10.2] 岁;346 [77.1%] 男性;平均 [SD] 基线 PANSS 评分,89.8 [10.3];平均 [SD] 基线 CGI-S 评分,4.8 [0.6])。 在预先指定的改良意向治疗疗效分析(n = 435)中,42 mg 鲁美特哌隆满足了主要和关键的次要疗效目标,表明从基线到第 28 天,PANSS 总分(至少平方平均差 [LSMD],-4.2;95% CI,-7.8 至 -0.6;P = .02;效应大小 [ES],-0.3)和 CGI-S(LSMD,-0.3;95% CI,- 0.5 至 -0.1;P = .003;ES,-0.4)。对于 28 mg 鲁美哌隆,从基线到第 28 天的 LSMD 为 -2.6(95% CI,-6.2 至 1.1;P = .16;ES,-0.2),PANSS 总分为 -0.2(95% CI,- 0.5 至 0.0;P = .02;ES,-0.3) 在 CGI-S 上。与安慰剂相比,两种鲁美特哌隆剂量均具有良好的耐受性,没有临床上显着的治疗引起的运动不良反应或心脏代谢或内分泌因素的变化。结论和相关性 Lumateperone 可有效改善精神分裂症症状,并具有良好的安全性。试验注册 ClinicalTrials.gov 标识符:NCT02282761。











































 京公网安备 11010802027423号
京公网安备 11010802027423号