当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly Efficient Extraction of Trivalent f‐Cations Using Several N‐Pivot Tripodal Diglycolamide Ligands in an Ionic Liquid: The Role of Ligand Structure on Metal Ion Complexation
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2020-01-08 , DOI: 10.1002/ejic.201900956 Seraj A. Ansari 1 , Prasanta K. Mohapatra 1 , Parveen K. Verma 1 , Andrea Leoncini 2 , Ashok K. Yadav 3 , Shambhu N. Jha 3 , Dibyendu Bhattacharyya 3 , Jurriaan Huskens 2 , Willem Verboom 2
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2020-01-08 , DOI: 10.1002/ejic.201900956 Seraj A. Ansari 1 , Prasanta K. Mohapatra 1 , Parveen K. Verma 1 , Andrea Leoncini 2 , Ashok K. Yadav 3 , Shambhu N. Jha 3 , Dibyendu Bhattacharyya 3 , Jurriaan Huskens 2 , Willem Verboom 2
Affiliation
|
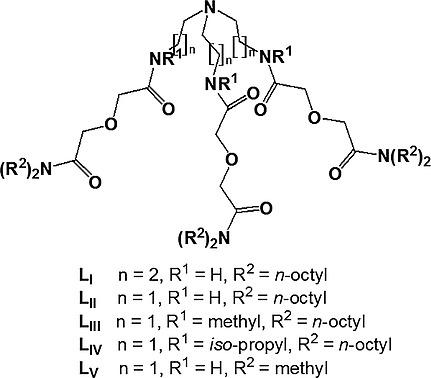
Complexation of trivalent lanthanides and actinides was studied in a room temperature ionic liquid (RTIL) using five N‐pivot tripodal diglycolamide ligands (LI–LV) having different structural features, viz. spacer lengths and substituents. The nature of the metal/Ligand complex changed with the substitution of N atom near the tripodal platform from ML with LIII and LIV to ML2 with LI, LII and LV (where M = Eu3+/Nd3+/Am3+). With 0.2 mmol/L ligands, the distribution ratio of Am3+ at 1 m HNO3 followed the order: LI (210) > LII (42) > LIII (2.1) > LV (0.7) > LIV (0.2). The formation constants between Nd3+ and the ligands (log βML,ML2) followed an identical order, viz. LI > LII > LIII > LV > LIV. The extraction of Am3+ or Eu3+ with all the ligands followed a cation‐exchange extraction mechanism, which is in sharp contrast to the results obtained in a molecular solvent (n‐dodecane), where a solvation extraction mechanism prevalied. Luminescence studies on the Eu3+ extract in RTIL confimed the absence of water molecules in the inner coordination sphere of the complex. EXAFS studies indicated that all three DGA arms were binding to the Eu3+ cation.
中文翻译:

在离子液体中使用几种N-轴三脚架二甘醇酰胺配体高效提取三价f-阳离子:配体结构对金属离子络合的作用
三价镧系元素和锕系元素的络合在使用五个室温离子液体(RTIL)进行了研究Ñ -pivot三脚二乙二醇配体(大号我-大号V具有不同的结构特征,即)。间隔长度和取代基。金属/配体配合物的性质随着三脚架平台附近的N原子的取代而从具有L III和L IV的ML变为具有L I,L II和L V的ML 2(其中M = Eu 3+ / Nd 3+ /上午3+)。在配体为0.2 mmol / L的情况下,Am 3+在1 m HNO 3上的分布比例为:L I(210)> L II(42)> L III(2.1)> L V(0.7)> L IV( 0.2)。钕之间的形成常数3+和配位体(对数 β ML,ML 2),然后一个相同的顺序,即 L I > L II > L III > L V > L IV。Am的提取具有所有配体的3+或Eu 3+遵循阳离子交换萃取机理,这与在分子溶剂(正十二烷)中获得溶剂化萃取机理的结果形成鲜明对比。在RTIL中对Eu 3+提取物进行的发光研究证实了该复合物内部配位域中不存在水分子。EXAFS研究表明,所有三个DGA臂均与Eu 3+阳离子结合。
更新日期:2020-01-08
中文翻译:

在离子液体中使用几种N-轴三脚架二甘醇酰胺配体高效提取三价f-阳离子:配体结构对金属离子络合的作用
三价镧系元素和锕系元素的络合在使用五个室温离子液体(RTIL)进行了研究Ñ -pivot三脚二乙二醇配体(大号我-大号V具有不同的结构特征,即)。间隔长度和取代基。金属/配体配合物的性质随着三脚架平台附近的N原子的取代而从具有L III和L IV的ML变为具有L I,L II和L V的ML 2(其中M = Eu 3+ / Nd 3+ /上午3+)。在配体为0.2 mmol / L的情况下,Am 3+在1 m HNO 3上的分布比例为:L I(210)> L II(42)> L III(2.1)> L V(0.7)> L IV( 0.2)。钕之间的形成常数3+和配位体(对数 β ML,ML 2),然后一个相同的顺序,即 L I > L II > L III > L V > L IV。Am的提取具有所有配体的3+或Eu 3+遵循阳离子交换萃取机理,这与在分子溶剂(正十二烷)中获得溶剂化萃取机理的结果形成鲜明对比。在RTIL中对Eu 3+提取物进行的发光研究证实了该复合物内部配位域中不存在水分子。EXAFS研究表明,所有三个DGA臂均与Eu 3+阳离子结合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号