当前位置:
X-MOL 学术
›
Lancet Diabetes Endocrinol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial.
The Lancet Diabetes & Endocrinology ( IF 44.0 ) Pub Date : 2020-01-07 , DOI: 10.1016/s2213-8587(19)30423-1 Hertzel C Gerstein,Robert Hart,Helen M Colhoun,Rafael Diaz,Mark Lakshmanan,Fady T Botros,Jeffrey Probstfield,Matthew C Riddle,Lars Rydén,Charles Messan Atisso,Leanne Dyal,Stephanie Hall,Alvaro Avezum,Jan Basile,Ignacio Conget,William C Cushman,Nicolae Hancu,Markolf Hanefeld,Petr Jansky,Matyas Keltai,Fernando Lanas,Lawrence A Leiter,Patricio Lopez-Jaramillo,Ernesto Germán Cardona Muñoz,Nana Pogosova,Peter J Raubenheimer,Jonathan E Shaw,Wayne H-H Sheu,Theodora Temelkova-Kurktschiev
The Lancet Diabetes & Endocrinology ( IF 44.0 ) Pub Date : 2020-01-07 , DOI: 10.1016/s2213-8587(19)30423-1 Hertzel C Gerstein,Robert Hart,Helen M Colhoun,Rafael Diaz,Mark Lakshmanan,Fady T Botros,Jeffrey Probstfield,Matthew C Riddle,Lars Rydén,Charles Messan Atisso,Leanne Dyal,Stephanie Hall,Alvaro Avezum,Jan Basile,Ignacio Conget,William C Cushman,Nicolae Hancu,Markolf Hanefeld,Petr Jansky,Matyas Keltai,Fernando Lanas,Lawrence A Leiter,Patricio Lopez-Jaramillo,Ernesto Germán Cardona Muñoz,Nana Pogosova,Peter J Raubenheimer,Jonathan E Shaw,Wayne H-H Sheu,Theodora Temelkova-Kurktschiev
|
BACKGROUND
Cardiovascular outcome trials have suggested that glucagon-like peptide 1 (GLP-1) receptor agonists might reduce strokes. We analysed the effect of dulaglutide on stroke within the researching cardiovascular events with a weekly incretin in diabetes (REWIND) trial.
METHODS
REWIND was a multicentre, randomised, double-blind, placebo-controlled trial done at 371 sites in 24 countries. Men and women (aged ≥50 years) with established or newly detected type 2 diabetes whose HbA1c was 9·5% or less (with no lower limit) on stable doses of up to two oral glucose-lowering drugs with or without basal insulin therapy were eligible if their body-mass index was at least 23 kg/m2. Participants were randomly assigned (1:1) to weekly subcutaneous injections of either masked dulaglutide 1·5 mg or the same volume of masked placebo (containing the same excipients but without dulaglutide). Randomisation was done by a computer-generated random code with an interactive web response system with stratification by site. Participants, investigators, the trial leadership, and all other personnel were masked to treatment allocation until the trial was completed and the database was locked. During the treatment period, participants in both groups were instructed to inject study drug on the same day at around the same time, each week. Strokes were categorised as fatal or non-fatal, and as either ischaemic, haemorrhagic, or undetermined. Stroke severity was assessed using the modified Rankin scale. Participants were seen at 2 weeks, 3 months, 6 months, and then every 3 months for drug dispensing and every 6 months for detailed assessments, until 1200 confirmed primary outcomes accrued. The primary endpoint was the first occurrence of any component of the composite outcome, which comprised non-fatal myocardial infarction, non-fatal stroke, or death from cardiovascular or unknown causes. All analyses were done according to an intention-to-treat strategy that included all randomly assigned participants, irrespective of adherence. The trial is registered with ClinicalTrials.gov, number NCT01394952.
FINDINGS
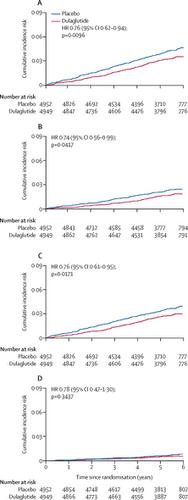
Between Aug 18, 2011, and Aug 14, 2013, we screened 12 133 patients, of whom 9901 with type 2 diabetes and additional cardiovascular risk factors were randomly assigned to either dulaglutide (n=4949) or an equal volume of placebo (n=4952). During a median follow-up of 5·4 years, cerebrovascular and other cardiovascular outcomes were ascertained and adjudicated. 158 (3·2%) of 4949 participants assigned to dulaglutide and 205 (4·1%) of 4952 participants assigned to placebo had a stroke during follow-up (hazard ratio [HR] 0·76, 95% CI 0·62-0·94; p=0·010). Dulaglutide reduced ischaemic stroke (0·75, 0·59-0·94, p=0·012) but had no effect on haemorrhagic stroke (1·05, 0·55-1·99; p=0·89). Dulaglutide also reduced the composite of non-fatal stroke or all-cause death (0·88, 0·79-0·98; p=0·017) and disabling stroke (0·74, 0·56-0·99; p=0·042). The degree of disability after stroke did not differ by treatment group.
INTERPRETATION
Long-term dulaglutide use might reduce clinically relevant ischaemic stroke in people with type 2 diabetes but does not affect stroke severity.
FUNDING
Eli Lilly and Company.
中文翻译:

地拉鲁肽对中风的影响:REWIND试验的探索性分析。
背景技术心血管结果试验表明,胰高血糖素样肽1(GLP-1)受体激动剂可能减少中风。我们通过每周一次的糖尿病肠降血糖素(REWIND)试验分析了研究心血管事件中的度拉鲁肽对中风的影响。方法REWIND是一项在24个国家/地区的371个地点进行的多中心,随机,双盲,安慰剂对照试验。患有或新发现的2型糖尿病的HbA1c为9·5%或更低(无下限)的男性和女性(≥50岁),采用稳定剂量的两种口服降糖药(有或没有基础胰岛素治疗)如果他们的身体质量指数至少为23 kg / m2,则符合资格。参与者被随机分配(1:1)每周一次皮下注射1×5 mg的经掩盖的dulaglutide或相同体积的经掩盖的安慰剂(含有相同的赋形剂,但不含dulaglutide)。随机化是通过计算机生成的随机代码和交互式Web响应系统按站点分层进行的。参与者,研究人员,试验领导者和所有其他人员都被掩盖了分配治疗的方法,直到试验完成并且数据库被锁定。在治疗期间,两组受试者均被指示在每周的大约同一时间,同一天注射研究药物。中风分为致命性或非致命性,分为缺血性,出血性或不确定性。使用改良的兰金量表评估卒中严重程度。在2周,3个月,6个月时看到参与者,然后每3个月进行一次药物分配,每6个月进行一次详细评估,直到获得1200个已确认的主要结局。主要终点是复合结局任何成分的首次出现,包括非致命性心肌梗塞,非致命性中风或因心血管原因或未知原因死亡。所有分析均根据意向治疗策略完成,该策略包括所有随机分配的参与者,无论是否依从。该试验已在ClinicalTrials.gov上注册,编号为NCT01394952。结果在2011年8月18日至2013年8月14日之间,我们筛选了12133例患者,其中9901型2型糖尿病和其他心血管危险因素被随机分配至dulaglutide(n = 4949)或等量安慰剂(n = 4952)。在5到4年的中位随访期间,确定并裁定脑血管和其他心血管结局。在随访期间,分配给dulaglutide的4949名参与者中有158名(3·2%),在分配给安慰剂的4952名参与者中205名(4·1%)有中风(危险比[HR] 0·76,95%CI 0·62 -0·94; p = 0·010)。杜拉鲁肽可降低缺血性卒中(0·75,0·59-0·94,p = 0·012),但对出血性卒中无影响(1·05,0·55-1·99; p = 0·89)。杜拉鲁肽还减少了非致命性卒中或全因死亡(0·88,0·79-0·98; p = 0·017)和致残性卒中(0·74,0·56-0·99; p = 0·042)。中风后的残疾程度在各治疗组中没有差异。解释长期使用度拉鲁肽可能会减少2型糖尿病患者的临床相关性缺血性中风,但不会影响中风的严重性。资金礼来公司。
更新日期:2020-01-22
中文翻译:

地拉鲁肽对中风的影响:REWIND试验的探索性分析。
背景技术心血管结果试验表明,胰高血糖素样肽1(GLP-1)受体激动剂可能减少中风。我们通过每周一次的糖尿病肠降血糖素(REWIND)试验分析了研究心血管事件中的度拉鲁肽对中风的影响。方法REWIND是一项在24个国家/地区的371个地点进行的多中心,随机,双盲,安慰剂对照试验。患有或新发现的2型糖尿病的HbA1c为9·5%或更低(无下限)的男性和女性(≥50岁),采用稳定剂量的两种口服降糖药(有或没有基础胰岛素治疗)如果他们的身体质量指数至少为23 kg / m2,则符合资格。参与者被随机分配(1:1)每周一次皮下注射1×5 mg的经掩盖的dulaglutide或相同体积的经掩盖的安慰剂(含有相同的赋形剂,但不含dulaglutide)。随机化是通过计算机生成的随机代码和交互式Web响应系统按站点分层进行的。参与者,研究人员,试验领导者和所有其他人员都被掩盖了分配治疗的方法,直到试验完成并且数据库被锁定。在治疗期间,两组受试者均被指示在每周的大约同一时间,同一天注射研究药物。中风分为致命性或非致命性,分为缺血性,出血性或不确定性。使用改良的兰金量表评估卒中严重程度。在2周,3个月,6个月时看到参与者,然后每3个月进行一次药物分配,每6个月进行一次详细评估,直到获得1200个已确认的主要结局。主要终点是复合结局任何成分的首次出现,包括非致命性心肌梗塞,非致命性中风或因心血管原因或未知原因死亡。所有分析均根据意向治疗策略完成,该策略包括所有随机分配的参与者,无论是否依从。该试验已在ClinicalTrials.gov上注册,编号为NCT01394952。结果在2011年8月18日至2013年8月14日之间,我们筛选了12133例患者,其中9901型2型糖尿病和其他心血管危险因素被随机分配至dulaglutide(n = 4949)或等量安慰剂(n = 4952)。在5到4年的中位随访期间,确定并裁定脑血管和其他心血管结局。在随访期间,分配给dulaglutide的4949名参与者中有158名(3·2%),在分配给安慰剂的4952名参与者中205名(4·1%)有中风(危险比[HR] 0·76,95%CI 0·62 -0·94; p = 0·010)。杜拉鲁肽可降低缺血性卒中(0·75,0·59-0·94,p = 0·012),但对出血性卒中无影响(1·05,0·55-1·99; p = 0·89)。杜拉鲁肽还减少了非致命性卒中或全因死亡(0·88,0·79-0·98; p = 0·017)和致残性卒中(0·74,0·56-0·99; p = 0·042)。中风后的残疾程度在各治疗组中没有差异。解释长期使用度拉鲁肽可能会减少2型糖尿病患者的临床相关性缺血性中风,但不会影响中风的严重性。资金礼来公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号