当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurement and Modeling of Excess Molar Enthalpies of Binary Mixtures Involving Hydrocarbon Components of Fuel
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jced.9b00949 Mohamed Lifi 1, 2 , Natalia Muñoz-Rujas 1 , Eduardo A. Montero 1 , Younes Chhiti 2, 3 , Fernando Aguilar 1 , Fatima Ezzahrae M’hamdi. Alaoui 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jced.9b00949 Mohamed Lifi 1, 2 , Natalia Muñoz-Rujas 1 , Eduardo A. Montero 1 , Younes Chhiti 2, 3 , Fernando Aguilar 1 , Fatima Ezzahrae M’hamdi. Alaoui 2
Affiliation
|
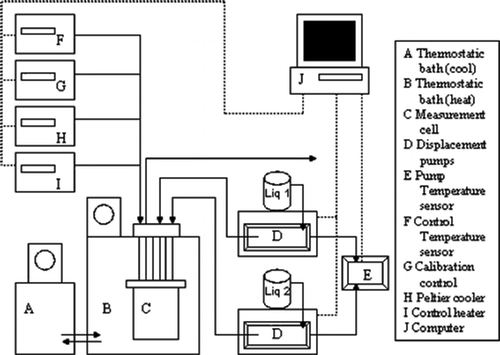
Experimental excess molar enthalpies of binary systems that are constituted by different groups of hydrocarbons, which are 1-hexene, methylcyclohexane, cyclohexane, isooctane, and heptane, are reported in this work. Precisely, the mixtures composed of 1-hexene, heptane, and isooctane were evaluated at 298.15 and 313.15 K although the rest of the mixtures were studied at 313.15 K. A quasi-isothermal flow calorimeter at atmospheric pressure has been used to measure the excess molar enthalpies of the studied mixtures. All the binary systems presented an endothermic behavior at the chosen temperatures. Furthermore, the measured data were fitted to the Redlich–Kister equation and NRTL and UNIQUAC models. Hence, the values of the standard deviation indicated the good agreement between the experimental data and the modeling results.
中文翻译:

涉及燃料碳氢化合物的二元混合物过量摩尔焓的测量和建模
在这项工作中,报道了由不同种类的烃组成的二元体系的实验过量摩尔焓,所述烃是1-己烯,甲基环己烷,环己烷,异辛烷和庚烷。精确地,由1-己烯,庚烷和异辛烷组成的混合物在298.15和313.15 K下进行了评估,尽管其余混合物在313.15 K下进行了研究。已经使用大气压下的准等温流量热仪来测量过剩的摩尔数。所研究混合物的焓。所有二元系统在所选温度下均表现出吸热行为。此外,将测量数据拟合到Redlich-Kister方程以及NRTL和UNIQUAC模型。因此,标准偏差的值表明实验数据与建模结果之间具有良好的一致性。
更新日期:2020-01-08
中文翻译:

涉及燃料碳氢化合物的二元混合物过量摩尔焓的测量和建模
在这项工作中,报道了由不同种类的烃组成的二元体系的实验过量摩尔焓,所述烃是1-己烯,甲基环己烷,环己烷,异辛烷和庚烷。精确地,由1-己烯,庚烷和异辛烷组成的混合物在298.15和313.15 K下进行了评估,尽管其余混合物在313.15 K下进行了研究。已经使用大气压下的准等温流量热仪来测量过剩的摩尔数。所研究混合物的焓。所有二元系统在所选温度下均表现出吸热行为。此外,将测量数据拟合到Redlich-Kister方程以及NRTL和UNIQUAC模型。因此,标准偏差的值表明实验数据与建模结果之间具有良好的一致性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号