当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Equilibrium for Binary Systems Containing Carboxylic Acids and Acetic Anhydride Obtained by Differential Scanning Calorimetry
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jced.9b00711 Jinghao He 1 , Dejun Dong 2 , Fan Yang 1 , Lihui Sun 2 , Xing Yu 2 , Zhanping Yang 2 , Xuedong Zhu 1 , Yinchun Liang 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jced.9b00711 Jinghao He 1 , Dejun Dong 2 , Fan Yang 1 , Lihui Sun 2 , Xing Yu 2 , Zhanping Yang 2 , Xuedong Zhu 1 , Yinchun Liang 2
Affiliation
|
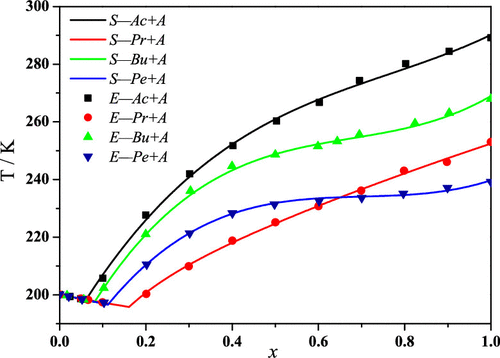
In the present study, the solid–liquid phase diagrams of four systems acetic acid + acetic anhydride, propionic acid + acetic anhydride, butyric acid + acetic anhydride, and pentanoic acid + acetic anhydride were determined by differential scanning calorimetry at atmospheric pressure. The experimental results illustrated that these systems showed a eutectic behavior. The experimental data were compared with predicted data acquired by solving the phase equilibrium equations using an algorithm. The liquid-phase activity coefficients were calculated by the Wilson and nonrandom two-liquid activity coefficient models. The results show that the approaches used for the calculated system equilibrium was capable to accurately describe the eutectic solid–liquid phase diagrams of the above-mentioned systems.
中文翻译:

差示扫描量热法获得的含有羧酸和乙酸酐的二元体系的固液平衡
在本研究中,通过差示扫描量热法在大气压下测定了四个体系的乙酸+乙酸酐,丙酸+乙酸酐,丁酸+乙酸酐和戊酸+乙酸酐的固液相图。实验结果表明,这些系统表现出共晶行为。将实验数据与通过使用算法求解相平衡方程而获得的预测数据进行比较。通过Wilson和非随机两液活度系数模型计算液相活度系数。结果表明,用于计算系统平衡的方法能够准确地描述上述系统的共晶固液相图。
更新日期:2020-01-08
中文翻译:

差示扫描量热法获得的含有羧酸和乙酸酐的二元体系的固液平衡
在本研究中,通过差示扫描量热法在大气压下测定了四个体系的乙酸+乙酸酐,丙酸+乙酸酐,丁酸+乙酸酐和戊酸+乙酸酐的固液相图。实验结果表明,这些系统表现出共晶行为。将实验数据与通过使用算法求解相平衡方程而获得的预测数据进行比较。通过Wilson和非随机两液活度系数模型计算液相活度系数。结果表明,用于计算系统平衡的方法能够准确地描述上述系统的共晶固液相图。











































 京公网安备 11010802027423号
京公网安备 11010802027423号