Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water Bridges in Clay Nanopores: Mechanisms of Formation and Impact on Hydrocarbon Transport.
Langmuir ( IF 3.7 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.langmuir.9b03244 Hao Xiong 1 , Deepak Devegowda 1 , Liangliang Huang 2
Langmuir ( IF 3.7 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.langmuir.9b03244 Hao Xiong 1 , Deepak Devegowda 1 , Liangliang Huang 2
Affiliation
|
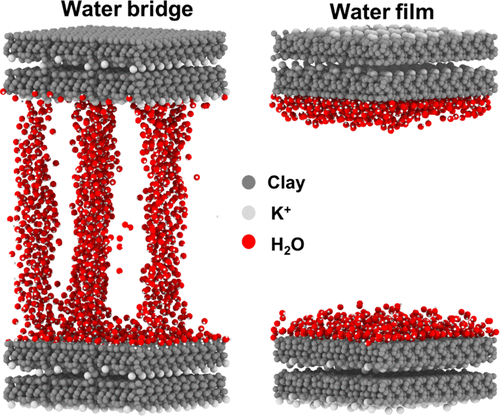
Clays are prevalent in the earth's crust and usually deposited in the presence of water. An unusual finding in clays is that under certain conditions, water molecules can collectively form a bridge across a clay-hosted pore. However, there are relatively few studies focused on the formation mechanism of the water bridge in clay nanopores. In this work, we use molecular dynamics simulations to investigate the formation of the water bridge and its influence on fluid transport in slit-shaped illite nanopores. Two different basal illite surface chemistries are constructed: potassium-hydroxyl (P-H) and hydroxyl-hydroxyl (H-H) structures. Because pore size and water concentration are expected to control the formation of the water bridge, our simulations span a wide range of pore sizes and water concentrations. Generally, positive potassium layers and negative hydroxyl groups in P-H nanopore can induce partial charges which in return produce instant and local electric fields, favoring the formation of the water bridge. In P-H nanopores, the water bridge happens at a relatively low water concentration. However, in H-H nanopores, the water bridge only forms at high water concentrations. Additionally, smaller pore sizes favor the formation of water bridges. However, the presence of an electric field promotes the formation of a water bridge even in larger pore sizes in P-H pores. The results also indicate that in both P-H and H-H nanopores, water adsorption films initially create a smooth surface to promote the hydrocarbon flow. In P-H nanopores, further increases in the water concentration causes a sharp decline in the self-diffusion coefficients of the hydrocarbon and water due to the formation of the water bridge. The presence of electric fields in P-H pores can however weaken the confinement effect of illite and promote the hydrocarbon flow. In contrast, in H-H nanopores, the self-diffusion coefficients decline slowly with the increase of water concentration. This is because no water bridge is formed at low water concentrations in H-H nanopores.
中文翻译:

粘土纳米孔中的水桥:形成机理和对烃输运的影响。
粘土普遍存在于地壳中,通常在水的存在下沉积。在粘土中的一个不寻常的发现是,在某些条件下,水分子可以共同形成跨越粘土孔隙的桥梁。然而,很少有研究关注粘土纳米孔中水桥的形成机理。在这项工作中,我们使用分子动力学模拟来研究水桥的形成及其对狭缝形伊利石纳米孔中流体传输的影响。构建了两种不同的基础伊利石基化学结构:钾-羟基(PH)和羟基-羟基(HH)结构。由于预计孔径和水浓度会控制水桥的形成,因此我们的模拟涵盖了很大范围的孔径和水浓度。通常,PH纳米孔中的正钾层和负羟基可诱导部分电荷,而这些电荷又会返回瞬时电场和局部电场,从而有利于水桥的形成。在PH纳米孔中,水桥发生在相对较低的水浓度下。但是,在HH纳米孔中,水桥仅在高水浓度下形成。另外,较小的孔径有利于水桥的形成。然而,即使在PH孔中较大的孔径下,电场的存在也会促进水桥的形成。结果还表明,在PH和HH纳米孔中,水吸附膜最初都会形成光滑的表面以促进烃的流动。在PH纳米孔中 由于水桥的形成,水浓度的进一步增加导致烃和水的自扩散系数急剧下降。但是,PH孔中电场的存在会削弱伊利石的约束作用并促进碳氢化合物的流动。相反,在HH纳米孔中,自扩散系数随水浓度的增加而缓慢下降。这是因为在HH纳米孔中低水浓度下不会形成水桥。
更新日期:2020-01-17
中文翻译:

粘土纳米孔中的水桥:形成机理和对烃输运的影响。
粘土普遍存在于地壳中,通常在水的存在下沉积。在粘土中的一个不寻常的发现是,在某些条件下,水分子可以共同形成跨越粘土孔隙的桥梁。然而,很少有研究关注粘土纳米孔中水桥的形成机理。在这项工作中,我们使用分子动力学模拟来研究水桥的形成及其对狭缝形伊利石纳米孔中流体传输的影响。构建了两种不同的基础伊利石基化学结构:钾-羟基(PH)和羟基-羟基(HH)结构。由于预计孔径和水浓度会控制水桥的形成,因此我们的模拟涵盖了很大范围的孔径和水浓度。通常,PH纳米孔中的正钾层和负羟基可诱导部分电荷,而这些电荷又会返回瞬时电场和局部电场,从而有利于水桥的形成。在PH纳米孔中,水桥发生在相对较低的水浓度下。但是,在HH纳米孔中,水桥仅在高水浓度下形成。另外,较小的孔径有利于水桥的形成。然而,即使在PH孔中较大的孔径下,电场的存在也会促进水桥的形成。结果还表明,在PH和HH纳米孔中,水吸附膜最初都会形成光滑的表面以促进烃的流动。在PH纳米孔中 由于水桥的形成,水浓度的进一步增加导致烃和水的自扩散系数急剧下降。但是,PH孔中电场的存在会削弱伊利石的约束作用并促进碳氢化合物的流动。相反,在HH纳米孔中,自扩散系数随水浓度的增加而缓慢下降。这是因为在HH纳米孔中低水浓度下不会形成水桥。









































 京公网安备 11010802027423号
京公网安备 11010802027423号