当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic NMR and Computational Studies Inform the Conformational Description of Dendrillane Terpenes from the Nudibranch Goniobranchus coi.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jnatprod.9b01051 Louise C Forster 1 , Gregory K Pierens 2 , Jack K Clegg 1 , Mary J Garson 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jnatprod.9b01051 Louise C Forster 1 , Gregory K Pierens 2 , Jack K Clegg 1 , Mary J Garson 1
Affiliation
|
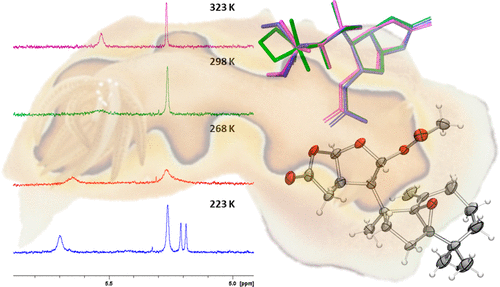
Two new oxygenated terpenes (1 and 2) have been characterized from the Australian nudibranch Goniobranchus coi. Broadened 1H NMR signals, together with the absence of individual carbon NMR signals, complicated analysis of 5,9-epoxydendrillolide A (1); increasing the temperature to 323 K revealed the missing NMR signals. Low-temperature 1H NMR experiments provided an activation barrier of ∼15 kcal mol-1 and, together with DFT calculations, supported interconversion of a twist chair conformer with two different chair conformers. X-ray crystallographic analysis coupled with biosynthetic reasoning suggested a (5R, 8S, 9R, 13R, 14R, 15R, 16R) configuration. Ketone 2 demonstrated similar dynamic conformational processes to 1.
中文翻译:

动态核磁共振和计算研究为来自 Nudibranch Goniobranchus coi 的 Dendrillane 萜烯的构象描述提供信息。
两种新的含氧萜烯(1 和 2)已从澳大利亚裸鳃亚目 Goniobranchus coi 中得到表征。加宽的 1H NMR 信号,加上没有单个碳 NMR 信号,5,9-环氧树胶内酯 A (1) 的复杂分析;将温度提高到 323 K 揭示了缺失的 NMR 信号。低温 1H NMR 实验提供了约 15 kcal mol-1 的激活势垒,并且结合 DFT 计算,支持扭曲椅子构象异构体与两种不同椅子构象异构体的互变。结合生物合成推理的 X 射线晶体学分析表明 (5R、8S、9R、13R、14R、15R、16R) 配置。酮 2 表现出与 1 相似的动态构象过程。
更新日期:2020-01-08
中文翻译:

动态核磁共振和计算研究为来自 Nudibranch Goniobranchus coi 的 Dendrillane 萜烯的构象描述提供信息。
两种新的含氧萜烯(1 和 2)已从澳大利亚裸鳃亚目 Goniobranchus coi 中得到表征。加宽的 1H NMR 信号,加上没有单个碳 NMR 信号,5,9-环氧树胶内酯 A (1) 的复杂分析;将温度提高到 323 K 揭示了缺失的 NMR 信号。低温 1H NMR 实验提供了约 15 kcal mol-1 的激活势垒,并且结合 DFT 计算,支持扭曲椅子构象异构体与两种不同椅子构象异构体的互变。结合生物合成推理的 X 射线晶体学分析表明 (5R、8S、9R、13R、14R、15R、16R) 配置。酮 2 表现出与 1 相似的动态构象过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号