当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stepwise Evolution of Fragment Hits against MAPK Interacting Kinases 1 and 2.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jmedchem.9b01582 Jacek Kwiatkowski 1 , Boping Liu 1 , Shermaine Pang 1 , Nur Huda Binte Ahmad 1 , Gang Wang 1 , Anders Poulsen 1 , Haiyan Yang 1 , Yong Rui Poh 1 , Doris Hui Ying Tee 1 , Esther Ong 1 , Priya Retna 1 , Nurul Dinie 1 , Perlyn Kwek 1 , John Liang Kuan Wee 1 , Vithya Manoharan 1 , Choon Bing Low 1 , Peck Gee Seah 1 , Vishal Pendharkar 1 , Kanda Sangthongpitag 1 , Joma Joy 1 , Nithya Baburajendran 1 , Anna Elisabet Jansson 1 , Kassoum Nacro 1 , Jeffrey Hill 1 , Thomas H Keller 1 , Alvin W Hung 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jmedchem.9b01582 Jacek Kwiatkowski 1 , Boping Liu 1 , Shermaine Pang 1 , Nur Huda Binte Ahmad 1 , Gang Wang 1 , Anders Poulsen 1 , Haiyan Yang 1 , Yong Rui Poh 1 , Doris Hui Ying Tee 1 , Esther Ong 1 , Priya Retna 1 , Nurul Dinie 1 , Perlyn Kwek 1 , John Liang Kuan Wee 1 , Vithya Manoharan 1 , Choon Bing Low 1 , Peck Gee Seah 1 , Vishal Pendharkar 1 , Kanda Sangthongpitag 1 , Joma Joy 1 , Nithya Baburajendran 1 , Anna Elisabet Jansson 1 , Kassoum Nacro 1 , Jeffrey Hill 1 , Thomas H Keller 1 , Alvin W Hung 1
Affiliation
|
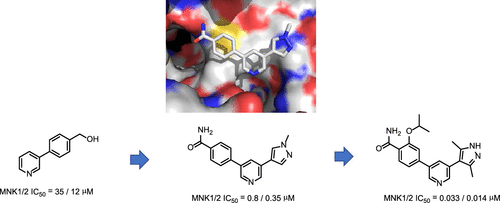
Dysregulation of translation initiation factor 4E (eIF4E) activity occurs in various cancers. Mitogen-activated protein kinase (MAPK) interacting kinases 1 and 2 (MNK1 and MNK2) play a fundamental role in activation of eIF4E. Structure-activity relationship-driven expansion of a fragment hit led to discovery of dual MNK1 and MNK2 inhibitors based on a novel pyridine-benzamide scaffold. The compounds possess promising in vitro and in vivo pharmacokinetic profiles and show potent on target inhibition of eIF4E phosphorylation in cells.
中文翻译:

MAPK相互作用激酶1和2的片段命中的逐步演变。
翻译起始因子4E(eIF4E)活性的异常调节发生在各种癌症中。丝裂素活化蛋白激酶(MAPK)相互作用的激酶1和2(MNK1和MNK2)在eIF4E的激活中起着基本作用。结构活性关系驱动片段命中的扩展导致基于新型吡啶-苯甲酰胺支架的双重MNK1和MNK2抑制剂的发现。这些化合物具有良好的体外和体内药代动力学特性,并且对细胞中eIF4E磷酸化的靶标抑制作用表现出强大的作用。
更新日期:2020-01-08
中文翻译:

MAPK相互作用激酶1和2的片段命中的逐步演变。
翻译起始因子4E(eIF4E)活性的异常调节发生在各种癌症中。丝裂素活化蛋白激酶(MAPK)相互作用的激酶1和2(MNK1和MNK2)在eIF4E的激活中起着基本作用。结构活性关系驱动片段命中的扩展导致基于新型吡啶-苯甲酰胺支架的双重MNK1和MNK2抑制剂的发现。这些化合物具有良好的体外和体内药代动力学特性,并且对细胞中eIF4E磷酸化的靶标抑制作用表现出强大的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号