当前位置:
X-MOL 学术
›
Nanoscale Adv.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Does gold behaves as hydrogen? A joint theoretical and experimental study
Nanoscale Advances ( IF 4.6 ) Pub Date : 2020/01/07 , DOI: 10.1039/c9na00780f Zhengbo Qin 1 , Jiangle Zhang 1, 2 , Chen Wang 1 , Lin Wang 1 , Zichao Tang 2
Nanoscale Advances ( IF 4.6 ) Pub Date : 2020/01/07 , DOI: 10.1039/c9na00780f Zhengbo Qin 1 , Jiangle Zhang 1, 2 , Chen Wang 1 , Lin Wang 1 , Zichao Tang 2
Affiliation
|
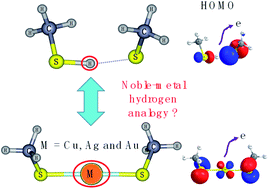
It has been established that the noble-metal–H analogue has been found in a large number of noble-metal–ligand clusters in view of geometric and electronic structures. Here, we demonstrated a different view of noble-metal–H analogue between noble-metal and hydrogen in M(SCH3)2− (M = Cu, Ag, Au and H) systems. Although H(SCH3)2− is a typical ion-hydrogen bonding cluster dramatically different from the chemical bonding clusters of M(SCH3)2− (M = Cu, Ag and Au), the comparison of the two typical bonding patterns has not yet been fully investigated. Through a series of chemical bonding analyses, it is indicated that the evolution has been exhibited from typical ionic bonding in Cu(SCH3)2− to a significant covalent bonding nature in Au(SCH3)2− and hydrogen bonding dominating in H(SCH3)2−. The comparison of M(SCH3)2− (M = Cu, Ag and Au) with H(SCH3)2− illustrates the differences in bonding between noble metals and hydrogen, which are mainly related to their diverse atomic orbitals participating in chemical bonding.
中文翻译:

黄金的行为与氢一样吗?联合理论和实验研究
从几何和电子结构来看,已经确定在大量贵金属-配体簇中发现了贵金属-H类似物。在这里,我们展示了 M(SCH 3 ) 2 − (M = Cu、Ag、Au 和 H) 体系中贵金属与氢之间的贵金属-H 类似物的不同观点。虽然H(SCH 3 ) 2 −是典型的离子氢键簇,与M(SCH 3 ) 2 − (M = Cu、Ag 和Au)的化学键簇有很大不同,但两种典型键合模式的比较表明:尚未得到充分调查。通过一系列的化学键合分析,表明Cu(SCH 3 ) 2 −中典型的离子键演化为Au(SCH 3 ) 2 −中的显着共价键性质以及H( 中氢键占主导地位)。 SCH 3 ) 2 − . M(SCH 3 ) 2 − (M = Cu、Ag 和 Au) 与 H(SCH 3 ) 2 −的比较说明了贵金属和氢之间成键的差异,这主要与其参与化学反应的原子轨道不同有关。粘合。
更新日期:2020-02-19
中文翻译:

黄金的行为与氢一样吗?联合理论和实验研究
从几何和电子结构来看,已经确定在大量贵金属-配体簇中发现了贵金属-H类似物。在这里,我们展示了 M(SCH 3 ) 2 − (M = Cu、Ag、Au 和 H) 体系中贵金属与氢之间的贵金属-H 类似物的不同观点。虽然H(SCH 3 ) 2 −是典型的离子氢键簇,与M(SCH 3 ) 2 − (M = Cu、Ag 和Au)的化学键簇有很大不同,但两种典型键合模式的比较表明:尚未得到充分调查。通过一系列的化学键合分析,表明Cu(SCH 3 ) 2 −中典型的离子键演化为Au(SCH 3 ) 2 −中的显着共价键性质以及H( 中氢键占主导地位)。 SCH 3 ) 2 − . M(SCH 3 ) 2 − (M = Cu、Ag 和 Au) 与 H(SCH 3 ) 2 −的比较说明了贵金属和氢之间成键的差异,这主要与其参与化学反应的原子轨道不同有关。粘合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号