当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigating Protein–Nanocrystal Interactions for Photodriven Activity
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-01-16 , DOI: 10.1021/acsabm.9b01025 Alexander W Harris , Albert Harguindey , Ryan E Patalano , Shambojit Roy , Omer Yehezkeli 1 , Andrew P Goodwin , Jennifer N Cha
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-01-16 , DOI: 10.1021/acsabm.9b01025 Alexander W Harris , Albert Harguindey , Ryan E Patalano , Shambojit Roy , Omer Yehezkeli 1 , Andrew P Goodwin , Jennifer N Cha
Affiliation
|
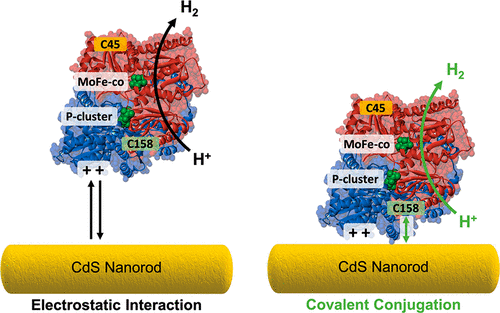
We illustrate how intermolecular interactions facilitate ATP-free electron transfer between either native or engineered MoFe protein (MoFeP) from nitrogenase and a CdS nanorod (NR) by following the reduction of H+ to H2. First, by varying the charge on the surface of the NR, we show the role of electrostatic interactions on MoFeP binding to the particle surface and subsequent H+ reduction. Next, the role of strong, semicovalent thiol–CdS interactions was tested using free cysteines on the MoFeP. By blocking free cysteines, we show that the presence of free thiols on the protein has little to no influence on CdS binding and resultant photocatalytic activity. We next studied methods to covalently bind the protein to CdS by modifying the free cysteines with dibenzocyclooctyne (DBCO) and reacting the CdS NRs capped with a mixture of negatively charged thioglycolic acid and thiol-PEG3-azide ligands. As compared to that of the unmodified proteins, a 32.2 ± 1.5% and 61.7 ± 2.1% increase in H2 production was observed from MoFeP and C-MoFeP, respectively. At last, to test the effect of both charge and covalent tethering, positively charged cysteamine/azide CdS NRs were reacted with DBCO-modified C-MoFeP, which showed little improvement over native C-MoFeP alone under irradiation. These results show the importance of both electrostatic associations between the NR and protein and covalently tethering the protein to the semiconductor surface for enhanced electron transfer and photodriven activity.
中文翻译:

研究光驱动活动的蛋白质-纳米晶体相互作用
我们通过将 H +还原为H 2来说明分子间相互作用如何促进来自固氮酶的天然或工程化 MoFe 蛋白 (MoFeP) 和 CdS 纳米棒 (NR) 之间的无 ATP 电子转移。首先,通过改变 NR 表面的电荷,我们展示了静电相互作用对 MoFeP 与颗粒表面的结合以及随后的 H +的作用。减少。接下来,使用 MoFeP 上的游离半胱氨酸测试强半共价硫醇-CdS 相互作用的作用。通过阻断游离半胱氨酸,我们表明蛋白质上游离硫醇的存在对 CdS 结合和由此产生的光催化活性几乎没有影响。我们接下来研究了通过用二苯并环辛炔 (DBCO) 修饰游离半胱氨酸并使 CdS NR 与带负电荷的巯基乙酸和硫醇-PEG3-叠氮化物配体的混合物反应来将蛋白质与 CdS 共价结合的方法。与未修饰的蛋白质相比,H 2增加了 32.2 ± 1.5% 和 61.7 ± 2.1%分别从 MoFeP 和 C-MoFeP 观察到生产。最后,为了测试电荷和共价束缚的效果,带正电荷的半胱胺/叠氮化物 CdS NRs 与 DBCO 修饰的 C-MoFeP 反应,在辐照下与单独的天然 C-MoFeP 相比几乎没有改善。这些结果表明 NR 和蛋白质之间的静电结合以及将蛋白质共价连接到半导体表面以增强电子转移和光驱动活性的重要性。
更新日期:2020-01-17
中文翻译:

研究光驱动活动的蛋白质-纳米晶体相互作用
我们通过将 H +还原为H 2来说明分子间相互作用如何促进来自固氮酶的天然或工程化 MoFe 蛋白 (MoFeP) 和 CdS 纳米棒 (NR) 之间的无 ATP 电子转移。首先,通过改变 NR 表面的电荷,我们展示了静电相互作用对 MoFeP 与颗粒表面的结合以及随后的 H +的作用。减少。接下来,使用 MoFeP 上的游离半胱氨酸测试强半共价硫醇-CdS 相互作用的作用。通过阻断游离半胱氨酸,我们表明蛋白质上游离硫醇的存在对 CdS 结合和由此产生的光催化活性几乎没有影响。我们接下来研究了通过用二苯并环辛炔 (DBCO) 修饰游离半胱氨酸并使 CdS NR 与带负电荷的巯基乙酸和硫醇-PEG3-叠氮化物配体的混合物反应来将蛋白质与 CdS 共价结合的方法。与未修饰的蛋白质相比,H 2增加了 32.2 ± 1.5% 和 61.7 ± 2.1%分别从 MoFeP 和 C-MoFeP 观察到生产。最后,为了测试电荷和共价束缚的效果,带正电荷的半胱胺/叠氮化物 CdS NRs 与 DBCO 修饰的 C-MoFeP 反应,在辐照下与单独的天然 C-MoFeP 相比几乎没有改善。这些结果表明 NR 和蛋白质之间的静电结合以及将蛋白质共价连接到半导体表面以增强电子转移和光驱动活性的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号