当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkyl Group Migration in Ni-Catalyzed Conjunctive Coupling with C(sp3) Electrophiles: Reaction Development and Application to Targets of Interest.
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.orglett.9b04453 Seung Moh Koo 1 , Alex J Vendola 1 , Sarah Noemi Momm 1 , James P Morken 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.orglett.9b04453 Seung Moh Koo 1 , Alex J Vendola 1 , Sarah Noemi Momm 1 , James P Morken 1
Affiliation
|
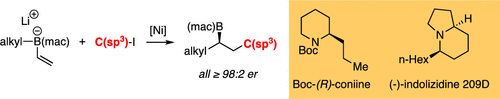
A catalytic conjunctive cross-coupling reaction is developed that allows the construction of chiral organoboronic esters from alkylboron ate complexes and alkyl iodide electrophiles. The process occurs most efficiently with a Ni/Pybox-comprised catalyst and with an acenapthoquinone-derived boron ligand. Because of the broad functional group tolerance of this reaction, it can be a versatile tool for organic synthesis. Applications to the construction of (R)-coniine and (-)-indolizidine 209D are described.
中文翻译:

Ni 催化与 C(sp3) 亲电子试剂的联合偶联中的烷基迁移:反应开发及其在感兴趣目标上的应用。
开发了一种催化联合交叉偶联反应,该反应允许从烷基硼酸酯配合物和烷基碘亲电子试剂构建手性有机硼酸酯。使用包含Ni/Pybox的催化剂和苊醌衍生的硼配体,该过程最有效地发生。由于该反应具有广泛的官能团耐受性,因此它可以成为有机合成的通用工具。描述了 (R)-coniine 和 (-)-indolizidine 209D 的构建应用。
更新日期:2020-01-07
中文翻译:

Ni 催化与 C(sp3) 亲电子试剂的联合偶联中的烷基迁移:反应开发及其在感兴趣目标上的应用。
开发了一种催化联合交叉偶联反应,该反应允许从烷基硼酸酯配合物和烷基碘亲电子试剂构建手性有机硼酸酯。使用包含Ni/Pybox的催化剂和苊醌衍生的硼配体,该过程最有效地发生。由于该反应具有广泛的官能团耐受性,因此它可以成为有机合成的通用工具。描述了 (R)-coniine 和 (-)-indolizidine 209D 的构建应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号