当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Thermoresponsive Micellar Assembly Constructed from a Hexameric Hemoprotein Modified with Poly(N-isopropylacrylamide) toward an Artificial Light-harvesting System
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-06 , DOI: 10.1021/jacs.9b10080 Shota Hirayama , Koji Oohora , Takayuki Uchihashi 1, 2 , Takashi Hayashi
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-06 , DOI: 10.1021/jacs.9b10080 Shota Hirayama , Koji Oohora , Takayuki Uchihashi 1, 2 , Takashi Hayashi
Affiliation
|
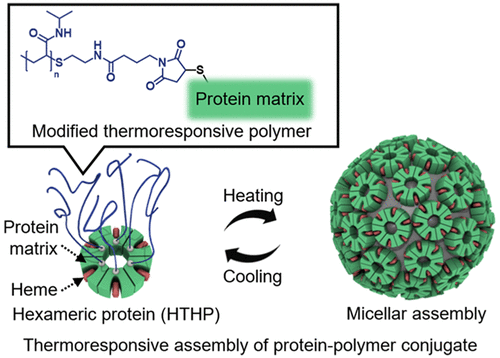
Artificial protein assemblies inspired by nature have significant potential in development of emergent functional materials. In order to construct an artificial protein assembly, we employed a mutant of a thermostable hemoprotein, hexameric tyrosine-coordinated heme protein (HTHP), as a building block. The HTHP mutant which has cysteine residues introduced on the bottom surface of its columnar structure was reacted with maleimide-tethering thermoresponsive poly(N-isopropylacrylamide), PNIPAAm, to generate the protein assembly upon heating. The site-specific modification of the cysteine residues with PNIPAAm on the protein surface was confirmed by SDS-PAGE and analytical size exclusion chromatography (SEC). The PNIPAAm-modified HTHP (PNIPAAm-HTHP) is found to provide a 43-nm spherical structure at 60 ºC and the structural changes observed between the assembled and disassembled forms were duplicated at least five-times. High-speed atomic force microscopic measurements of the micellar assembly supported by cross-linkage with glutaraldehyde indicates that the protein matrices are located on the surface of the sphere and cover the inner PNIPAAm core. Furthermore, substitution of heme with a photosensitizer, Zn protoporphyrin IX (ZnPP) in the micellar assembly provides an artificial light-harvesting system. Photochemical measurements of the ZnPP-substituted micellar assembly demonstrate that energy migration among the arrayed ZnPP molecules occurs within the range of several tens of picoseconds. Our present work represents the first example of an artificial light-harvesting system based on an assembled hemoprotein oligomer structure to replicate natural light-harvesting systems.
中文翻译:

由聚(N-异丙基丙烯酰胺)修饰的六聚血红蛋白构建的热响应胶束组件朝向人工光捕获系统
受自然启发的人工蛋白质组装体在新兴功能材料的开发中具有巨大潜力。为了构建人工蛋白质组件,我们采用了一种热稳定血红素蛋白、六聚酪氨酸协调血红素蛋白 (HTHP) 的突变体作为构建块。在其柱状结构的底部表面引入了半胱氨酸残基的 HTHP 突变体与马来酰亚胺系链热响应性聚(N-异丙基丙烯酰胺)PNIPAAm 反应,以在加热时产生蛋白质组装体。通过 SDS-PAGE 和分析尺寸排阻色谱 (SEC) 确认了蛋白质表面上半胱氨酸残基与 PNIPAAm 的位点特异性修饰。发现 PNIPAAm 修饰的 HTHP (PNIPAAm-HTHP) 在 60 ºC 下提供 43 nm 的球形结构,并且在组装和拆卸形式之间观察到的结构变化至少重复了五次。由戊二醛交联支持的胶束组件的高速原子力显微测量表明蛋白质基质位于球体表面并覆盖内部 PNIPAAm 核心。此外,在胶束组件中用光敏剂锌原卟啉 IX (ZnPP) 替代血红素提供了一种人工光收集系统。ZnPP 取代胶束组件的光化学测量表明,排列的 ZnPP 分子之间的能量迁移发生在几十皮秒的范围内。
更新日期:2020-01-06
中文翻译:

由聚(N-异丙基丙烯酰胺)修饰的六聚血红蛋白构建的热响应胶束组件朝向人工光捕获系统
受自然启发的人工蛋白质组装体在新兴功能材料的开发中具有巨大潜力。为了构建人工蛋白质组件,我们采用了一种热稳定血红素蛋白、六聚酪氨酸协调血红素蛋白 (HTHP) 的突变体作为构建块。在其柱状结构的底部表面引入了半胱氨酸残基的 HTHP 突变体与马来酰亚胺系链热响应性聚(N-异丙基丙烯酰胺)PNIPAAm 反应,以在加热时产生蛋白质组装体。通过 SDS-PAGE 和分析尺寸排阻色谱 (SEC) 确认了蛋白质表面上半胱氨酸残基与 PNIPAAm 的位点特异性修饰。发现 PNIPAAm 修饰的 HTHP (PNIPAAm-HTHP) 在 60 ºC 下提供 43 nm 的球形结构,并且在组装和拆卸形式之间观察到的结构变化至少重复了五次。由戊二醛交联支持的胶束组件的高速原子力显微测量表明蛋白质基质位于球体表面并覆盖内部 PNIPAAm 核心。此外,在胶束组件中用光敏剂锌原卟啉 IX (ZnPP) 替代血红素提供了一种人工光收集系统。ZnPP 取代胶束组件的光化学测量表明,排列的 ZnPP 分子之间的能量迁移发生在几十皮秒的范围内。











































 京公网安备 11010802027423号
京公网安备 11010802027423号