当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dimerization of Aldehydes into Esters by an Octahedral d6-Rhodium cis-Dihydride Catalyst: Inner- versus Outer-Sphere Mechanisms
Organometallics ( IF 2.5 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.organomet.9b00622 Josephina Mallah 1 , Mohamad Ataya 1 , Faraj Hasanayn 1
Organometallics ( IF 2.5 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.organomet.9b00622 Josephina Mallah 1 , Mohamad Ataya 1 , Faraj Hasanayn 1
Affiliation
|
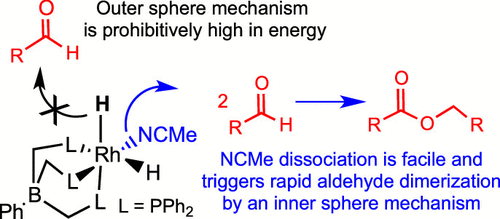
The tripodal ligated octahedral complex d6-[Rh(PhB(CH2PPh2)3)(H)2(NCMe)] (1-RhH) was discovered by Tejel and co-workers to catalyze the Tishchenko reaction in which two aldehydes are dimerized into an ester. Two fundamentally different mechanisms can be envisaged for this system: (i) an inner-sphere mechanism starting with substitution of the acetonitrile ligand of 1-RhH by an aldehyde and (ii) an outer-sphere mechanism starting with direct insertion of an aldehyde into a Rh–H bond of the intact 1-RhH to make an octahedral Rh-alkoxide intermediate. We use DFT methods to investigate the two mechanisms. The inner-sphere mechanism is computed to be energetically favorable. The outer-sphere one, in contrast, is prohibitively high in energy. This is opposite to catalysis of the same reaction using Gusev’s pincer-ligated octahedral catalyst trans-[(PHNN)Os(H)2(CO)] (2-OsH) where the outer-sphere mechanism was previously reported to have very low energy. The different behaviors of 1-RhH and 2-OsH can be attributed to a role from the different metals in the two catalysts as well as a role from their different ligands. Specifically, the higher oxidation state of the metal in 1-RhH, Rh(III) versus Os(II), greatly diminishes its thermodynamic hydricity leading to separated ions compared to 2-OsH, whereas the amino functionality of the ligand in 2-OsH greatly favors the kinetic hydricity in the reaction with an aldehyde by hydrogen bonding with the carbonyl group being reduced. Comparisons are also made with Milstein’s trans-[PNN-Ru(H)2(CO)] alcohol dehydrogenative coupling catalyst (3-RuH) which also lacks the amino functionality.
中文翻译:

八面体d 6-铑-顺式-二氢化物催化剂将醛二聚为酯:内-外层机理
Tejel及其同事发现了三脚架连接的八面体络合物d 6- [Rh(PhB(CH 2 PPh 2)3)(H)2(NCMe)](1 -RhH)催化Tishchenko反应,其中两个醛被二聚为酯。对于该系统,可以设想两种根本不同的机理:(i)以醛取代1- RhH的乙腈配体开始的内球机理,和(ii)以直接将醛插入醛中的外球机理完整1的Rh–H键-RhH制备八面体Rh-醇盐中间体。我们使用DFT方法研究这两种机制。计算出内球机理在能量上是有利的。相比之下,外球体的能量高得惊人。这与使用Gusev的夹钳式八面体催化剂反式-[[(P H NN)Os(H)2(CO)](2 -OsH)催化相同的反应相反,以前据报道外层机理非常低能量。1- RhH和2- OsH的不同行为可归因于两种催化剂中不同金属的作用以及它们不同配体的作用。具体而言,金属中较高的氧化态1- RhH,Rh(III)与Os(II)相比于2- OsH大大降低了其热力学水合导致分离的离子,而2- OsH中配体的氨基官能团则极大地促进了反应的动力学水合。醛通过与羰基的氢键键合而还原。还与同样缺少氨基官能团的米尔斯坦反式-[PNN-Ru(H)2(CO)]醇脱氢偶联催化剂(3- RuH)进行了比较。
更新日期:2020-01-07
中文翻译:

八面体d 6-铑-顺式-二氢化物催化剂将醛二聚为酯:内-外层机理
Tejel及其同事发现了三脚架连接的八面体络合物d 6- [Rh(PhB(CH 2 PPh 2)3)(H)2(NCMe)](1 -RhH)催化Tishchenko反应,其中两个醛被二聚为酯。对于该系统,可以设想两种根本不同的机理:(i)以醛取代1- RhH的乙腈配体开始的内球机理,和(ii)以直接将醛插入醛中的外球机理完整1的Rh–H键-RhH制备八面体Rh-醇盐中间体。我们使用DFT方法研究这两种机制。计算出内球机理在能量上是有利的。相比之下,外球体的能量高得惊人。这与使用Gusev的夹钳式八面体催化剂反式-[[(P H NN)Os(H)2(CO)](2 -OsH)催化相同的反应相反,以前据报道外层机理非常低能量。1- RhH和2- OsH的不同行为可归因于两种催化剂中不同金属的作用以及它们不同配体的作用。具体而言,金属中较高的氧化态1- RhH,Rh(III)与Os(II)相比于2- OsH大大降低了其热力学水合导致分离的离子,而2- OsH中配体的氨基官能团则极大地促进了反应的动力学水合。醛通过与羰基的氢键键合而还原。还与同样缺少氨基官能团的米尔斯坦反式-[PNN-Ru(H)2(CO)]醇脱氢偶联催化剂(3- RuH)进行了比较。











































 京公网安备 11010802027423号
京公网安备 11010802027423号