当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Styryllactones from Goniothalamus tamirensis
Phytochemistry ( IF 3.2 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.phytochem.2019.112248 Pornphimol Meesakul 1 , Wuttichai Jaidee 2 , Christopher Richardson 3 , Raymond J Andersen 4 , Brian O Patrick 4 , Anthony C Willis 5 , Chatchai Muanprasat 6 , Jin Wang 7 , Xiaoguang Lei 8 , Sarinya Hadsadee 9 , Siriporn Jungsuttiwong 9 , Stephen G Pyne 3 , Surat Laphookhieo 2
Phytochemistry ( IF 3.2 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.phytochem.2019.112248 Pornphimol Meesakul 1 , Wuttichai Jaidee 2 , Christopher Richardson 3 , Raymond J Andersen 4 , Brian O Patrick 4 , Anthony C Willis 5 , Chatchai Muanprasat 6 , Jin Wang 7 , Xiaoguang Lei 8 , Sarinya Hadsadee 9 , Siriporn Jungsuttiwong 9 , Stephen G Pyne 3 , Surat Laphookhieo 2
Affiliation
|
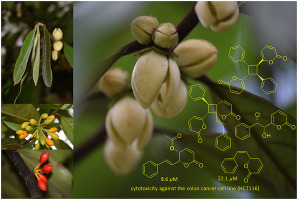
The phytochemical investigation of the twig and leaf extracts of Goniothalamus tamirensis led to the isolation and identification of 15 compounds including three rare previously undescribed styryllactones, goniotamirenones A-C, together with 12 known compounds. (Z)-6-Styryl-5,6-dihydro-2-pyranone and 5-(1-hydroxy-3-phenyl-allyl)-dihydro-furan-2-one are reported here for the first time as previously undescribed natural products. Their structures were elucidated by spectroscopic methods. Goniotamirenone A was synthesized via a [2 + 2] cycloaddition reaction of 6-styrrylpyran-2-one in quantitative yield. The absolute configurations of goniotamirenones B and C were identified from experimental and calculated ECD data, while the absolute configurations of (-)-5-acetoxygoniothalamin, (-)-isoaltholactone, parvistone E, and 5-(1-hydroxy-3-phenyl-allyl)-dihydro-furan-2-one were identified by single-crystal X-ray diffraction analysis using Cu Kα radiation. The absolute configurations of the other related compounds were determined from comparisons of their ECD spectra with relevant compounds reported in the literature. (-)-5-Acetoxygoniothalamin exhibited potent cytotoxicity against the colon cancer cell line (HCT116) with an IC50 value of 8.6 μM which was better than the standard control (doxorubicin, IC50 = 9.7 μM), while (Z)-6-styryl-5,6-dihydro-2-pyranone was less active with an IC50 value of 22.1 μM.
中文翻译:

来自 Goniothalamus tamirensis 的苯乙烯内酯
对 Goniothalamus tamirensis 的树枝和叶子提取物的植物化学研究导致了 15 种化合物的分离和鉴定,包括三种罕见的以前未描述的苯乙烯内酯,goniotamirenones AC,以及 12 种已知化合物。(Z)-6-Styryl-5,6-dihydro-2-pyranone 和 5-(1-hydroxy-3-phenyl-allyl)-dihydro-furan-2-one 在这里首次报道为以前未描述的天然产品。它们的结构是通过光谱方法阐明的。Goniotamirenone A 通过 6-styrrylpyran-2-one 的 [2 + 2] 环加成反应以定量收率合成。从实验和计算的 ECD 数据中确定了 goniotamirenones B 和 C 的绝对构型,而 (-)-5-acetoxygoniothalamin、(-)-isoaltholactone、parvistone E、和 5-(1-羟基-3-苯基-烯丙基)-二氢-呋喃-2-one 通过使用 Cu Kα 辐射的单晶 X 射线衍射分析进行鉴定。其他相关化合物的绝对构型是通过将它们的 ECD 光谱与文献中报道的相关化合物进行比较来确定的。(-)-5-Acetoxygoniothalamin 对结肠癌细胞系 (HCT116) 表现出有效的细胞毒性,IC50 值为 8.6 μM,优于标准对照(多柔比星,IC50 = 9.7 μM),而 (Z)-6-苯乙烯基-5,6-dihydro-2-pyranone 的活性较低,IC50 值为 22.1 μM。
更新日期:2020-03-01
中文翻译:

来自 Goniothalamus tamirensis 的苯乙烯内酯
对 Goniothalamus tamirensis 的树枝和叶子提取物的植物化学研究导致了 15 种化合物的分离和鉴定,包括三种罕见的以前未描述的苯乙烯内酯,goniotamirenones AC,以及 12 种已知化合物。(Z)-6-Styryl-5,6-dihydro-2-pyranone 和 5-(1-hydroxy-3-phenyl-allyl)-dihydro-furan-2-one 在这里首次报道为以前未描述的天然产品。它们的结构是通过光谱方法阐明的。Goniotamirenone A 通过 6-styrrylpyran-2-one 的 [2 + 2] 环加成反应以定量收率合成。从实验和计算的 ECD 数据中确定了 goniotamirenones B 和 C 的绝对构型,而 (-)-5-acetoxygoniothalamin、(-)-isoaltholactone、parvistone E、和 5-(1-羟基-3-苯基-烯丙基)-二氢-呋喃-2-one 通过使用 Cu Kα 辐射的单晶 X 射线衍射分析进行鉴定。其他相关化合物的绝对构型是通过将它们的 ECD 光谱与文献中报道的相关化合物进行比较来确定的。(-)-5-Acetoxygoniothalamin 对结肠癌细胞系 (HCT116) 表现出有效的细胞毒性,IC50 值为 8.6 μM,优于标准对照(多柔比星,IC50 = 9.7 μM),而 (Z)-6-苯乙烯基-5,6-dihydro-2-pyranone 的活性较低,IC50 值为 22.1 μM。











































 京公网安备 11010802027423号
京公网安备 11010802027423号