当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aminoalcoholate-driven tetracopper(II) cores as dual acetyl and butyrylcholinesterase inhibitors: Experimental and theoretical elucidation of mechanism of action.
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.jinorgbio.2019.110990 Aleksandra M Bondžić 1 , Milan V Senćanski 1 , Ana V Vujačić Nikezić 1 , Marina V Kirillova 2 , Vânia André 2 , Alexander M Kirillov 3 , Bojan P Bondžić 4
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.jinorgbio.2019.110990 Aleksandra M Bondžić 1 , Milan V Senćanski 1 , Ana V Vujačić Nikezić 1 , Marina V Kirillova 2 , Vânia André 2 , Alexander M Kirillov 3 , Bojan P Bondžić 4
Affiliation
|
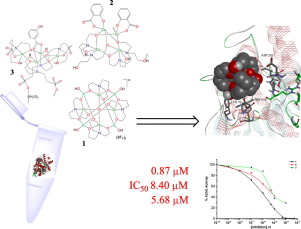
Three coordination compounds featuring different types of tetracopper(II) cores, namely [O ⊂ Cu4{N(CH2CH2O)3}4(BOH)4][BF4]2 (1), [Cu4(μ4-H2edte)(μ5-H2edte)(sal)2]n·7nH2O, (H4edte = N,N,N',N'-tetrakis(2-hydroxyethyl)ethylenediamine, H2sal = salicylic acid) (2), and [{Cu4(μ3-Hbes)4(μ-hba)}K(H2O)3]n, H3bes = N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (3), were assayed for their potency to inhibit the acetyl (AChE) and butyrylcholinesterase (BuChE) enzymes aiming to test these compounds as potential dual inhibitors in the treatment of Alzheimer's disease. All the investigated compounds showed a strong inhibitory potency toward both enzymes with IC50 values in micromolar range of concentration; compound 1 displayed the most potent inhibitory behaviour toward both enzymes. The mechanism of the AChE and BuChE inhibition was examined by enzyme kinetic measurements. The obtained kinetic parameters, Vmax and Km indicated an uncompetitive type of inhibition of both enzymes by compound 1. For the other two compounds a non-competitive inhibition mode was observed. To get further insight into the mechanism of action and to elucidate binding modes in details we examined the interactions of 1-3 with acetylcholinesterase, using molecular docking approach. Grid based docking studies indicated that these compounds can bind to peripheral anionic site (PAS) of the AChE with Ki values in micromolar range. Moreover, blind docking revealed the capability of investigated compounds to bind to new allosteric site (i.e. binding site II) distinct from PAS. Showing that these Cu-based compounds can act as new allosteric inhibitors of AChE and identifying novel allosteric binding site on AChE represents a significant contribution toward the design of novel and more effective inhibitors of AChE.
中文翻译:

氨基乙醇驱动的tetracopper(II)核心作为乙酰基和丁酰胆碱酯酶的双重抑制剂:作用机理的实验和理论解释。
具有不同类型的四铜(II)核的三种配位化合物,即[O⊂Cu4 {N(CH2CH2O)3} 4(BOH)4] [BF4] 2(1),[Cu4(μ4-H2edte)(μ5-H2edte) )(sal)2] n·7nH2O,(H4edte = N,N,N',N'-四(2-羟乙基)乙二胺,H2sal =水杨酸)(2)和[{Cu4(μ3-Hbes)4分析了(μ-hba)} K(H2O)3] n,H3bes = N,N-双(2-羟乙基)-2-氨基乙烷磺酸(3)抑制乙酰基(AChE)和丁酰胆碱酯酶的能力( BuChE)酶旨在测试这些化合物是否可作为治疗阿尔茨海默氏病的潜在双重抑制剂。所有研究的化合物对两种酶均表现出强大的抑制力,IC50值在微摩尔浓度范围内。化合物1对两种酶表现出最有效的抑制作用。通过酶动力学测量检查了AChE和BuChE抑制的机理。所获得的动力学参数Vmax和Km表明化合物1对两种酶的抑制作用均为非竞争性。对于其他两种化合物,观察到非竞争性抑制模式。为了进一步了解作用机理并详细阐明结合模式,我们使用分子对接方法研究了1-3与乙酰胆碱酯酶的相互作用。基于网格的对接研究表明,这些化合物可以与Ki值在微摩尔范围内的AChE的外围阴离子位点(PAS)结合。此外,盲对接揭示了所研究化合物结合不同于PAS的新变构位点(即结合位点II)的能力。
更新日期:2020-01-07
中文翻译:

氨基乙醇驱动的tetracopper(II)核心作为乙酰基和丁酰胆碱酯酶的双重抑制剂:作用机理的实验和理论解释。
具有不同类型的四铜(II)核的三种配位化合物,即[O⊂Cu4 {N(CH2CH2O)3} 4(BOH)4] [BF4] 2(1),[Cu4(μ4-H2edte)(μ5-H2edte) )(sal)2] n·7nH2O,(H4edte = N,N,N',N'-四(2-羟乙基)乙二胺,H2sal =水杨酸)(2)和[{Cu4(μ3-Hbes)4分析了(μ-hba)} K(H2O)3] n,H3bes = N,N-双(2-羟乙基)-2-氨基乙烷磺酸(3)抑制乙酰基(AChE)和丁酰胆碱酯酶的能力( BuChE)酶旨在测试这些化合物是否可作为治疗阿尔茨海默氏病的潜在双重抑制剂。所有研究的化合物对两种酶均表现出强大的抑制力,IC50值在微摩尔浓度范围内。化合物1对两种酶表现出最有效的抑制作用。通过酶动力学测量检查了AChE和BuChE抑制的机理。所获得的动力学参数Vmax和Km表明化合物1对两种酶的抑制作用均为非竞争性。对于其他两种化合物,观察到非竞争性抑制模式。为了进一步了解作用机理并详细阐明结合模式,我们使用分子对接方法研究了1-3与乙酰胆碱酯酶的相互作用。基于网格的对接研究表明,这些化合物可以与Ki值在微摩尔范围内的AChE的外围阴离子位点(PAS)结合。此外,盲对接揭示了所研究化合物结合不同于PAS的新变构位点(即结合位点II)的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号