当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Decoration of Selenide on Copper Foam for the Efficient Immobilization of Gaseous Elemental Mercury.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.est.9b07057 Jianping Yang 1 , Qin Li 1 , Min Li 1 , Wenbing Zhu 1 , Zequn Yang 2 , Wenqi Qu 1 , Yingchao Hu 1 , Hailong Li 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.est.9b07057 Jianping Yang 1 , Qin Li 1 , Min Li 1 , Wenbing Zhu 1 , Zequn Yang 2 , Wenqi Qu 1 , Yingchao Hu 1 , Hailong Li 1
Affiliation
|
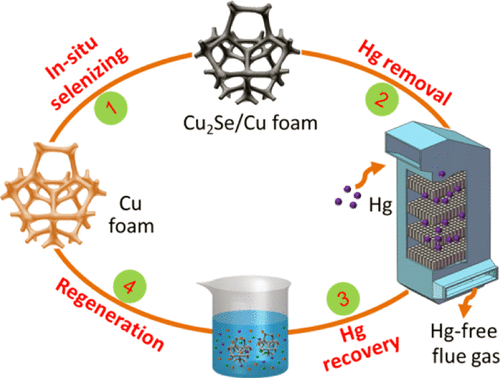
Mercury emission from industrial activities is a great threat to public health and ecosystems. Developing new strategies and materials to remove mercury still remains a serious task. Herein, selenide-decorated copper foam prepared by a heating-stirring method (Cu-hs) was used as a monolithic mercury adsorption material. The Cu-hs exhibited much better adsorption of elemental mercury (Hg0) compared with the selenide-decorated cordierite honeycomb prepared by the same method (Cordierite-hs). Nearly 100% Hg0 adsorption efficiency was obtained under a high gaseous hourly space velocity of 6.0 × 104. Excellent Hg0 adsorption capacity was obtained in a wide range of reaction temperatures from 40 to 120 °C, suggesting the good adaptability of Cu-hs in different operating conditions. The Cu-hs exhibited high selectivity for Hg0 against H2O and SO2, which is advantageous for real applications in industrial flue gas. The Hg0 adsorption capacity of Cu-hs reached 3743 g/m3, about 14 times higher than the 243 g/m3 of Cordierite-hs. The excellent Hg0 adsorption performance of Cu-hs was attributed to the high affinity of the selenium in Cu2Se for mercury, the homogeneous distribution of Cu2Se, and the superior fluid characteristics of the Cu foam substrate. The adsorption performance of the spent Cu-hs could be effectively recovered by HCl solution leaching and subsequent reselenization. The utilization of recyclable Cu-hs provides a cost-effective and environmentally friendly method for removing mercury from industrial flue gas.
中文翻译:

硒化物在铜泡沫上的原位装饰,可有效固定气态元素汞。
工业活动中的汞排放对公共卫生和生态系统构成了巨大威胁。研发去除汞的新策略和材料仍然是一项艰巨的任务。此处,将通过加热搅拌法(Cu-hs)制备的硒化物装饰的铜泡沫用作整体式汞吸附材料。与用相同方法制备的硒化物装饰的堇青石蜂窝相比,Cu-hs表现出更好的元素汞(Hg0)吸附。在6.0×104的高气体时空速下,Hg0的吸附效率接近100%。在40至120°C的宽反应温度范围内,均获得了优异的Hg0吸附能力,这表明Cu-hs在不同温度下具有良好的适应性运行条件。Cu-hs对Hg0对H2O和SO2具有很高的选择性,这对于在工业烟气中的实际应用是有利的。Cu-hs的Hg0吸附容量达到3743 g / m3,比堇青石-hs的243 g / m3高约14倍。Cu-hs优异的Hg0吸附性能归因于Cu2Se中硒对汞的高亲和力,Cu2Se的均匀分布以及Cu泡沫基材的优异流体特性。通过HCl溶液浸出和随后的再硒化可以有效地恢复废Cu-hs的吸附性能。可循环利用的铜-硫化氢的使用为从工业烟气中去除汞提供了一种经济高效且环保的方法。比堇青石-hs 243 g / m3高约14倍。Cu-hs优异的Hg0吸附性能归因于Cu2Se中硒对汞的高亲和力,Cu2Se的均匀分布以及Cu泡沫基材的优异流体特性。通过HCl溶液浸出和随后的再硒化可以有效地恢复废Cu-hs的吸附性能。可循环利用的铜-硫化氢的使用为从工业烟气中去除汞提供了一种经济高效且环保的方法。比堇青石-hs 243 g / m3高约14倍。Cu-hs优异的Hg0吸附性能归因于Cu2Se中硒对汞的高亲和力,Cu2Se的均匀分布以及Cu泡沫基材的优异流体特性。通过HCl溶液浸出和随后的再硒化可以有效地恢复废Cu-hs的吸附性能。可循环利用的铜-硫化氢的使用为从工业烟气中去除汞提供了一种经济高效且环保的方法。通过HCl溶液浸出和随后的再硒化可以有效地恢复废Cu-hs的吸附性能。可循环利用的铜-硫化氢的使用为从工业烟气中去除汞提供了一种经济高效且环保的方法。通过HCl溶液浸出和随后的再硒化可以有效地恢复废Cu-hs的吸附性能。可循环利用的铜-硫化氢的使用为从工业烟气中去除汞提供了一种经济高效且环保的方法。
更新日期:2020-01-15
中文翻译:

硒化物在铜泡沫上的原位装饰,可有效固定气态元素汞。
工业活动中的汞排放对公共卫生和生态系统构成了巨大威胁。研发去除汞的新策略和材料仍然是一项艰巨的任务。此处,将通过加热搅拌法(Cu-hs)制备的硒化物装饰的铜泡沫用作整体式汞吸附材料。与用相同方法制备的硒化物装饰的堇青石蜂窝相比,Cu-hs表现出更好的元素汞(Hg0)吸附。在6.0×104的高气体时空速下,Hg0的吸附效率接近100%。在40至120°C的宽反应温度范围内,均获得了优异的Hg0吸附能力,这表明Cu-hs在不同温度下具有良好的适应性运行条件。Cu-hs对Hg0对H2O和SO2具有很高的选择性,这对于在工业烟气中的实际应用是有利的。Cu-hs的Hg0吸附容量达到3743 g / m3,比堇青石-hs的243 g / m3高约14倍。Cu-hs优异的Hg0吸附性能归因于Cu2Se中硒对汞的高亲和力,Cu2Se的均匀分布以及Cu泡沫基材的优异流体特性。通过HCl溶液浸出和随后的再硒化可以有效地恢复废Cu-hs的吸附性能。可循环利用的铜-硫化氢的使用为从工业烟气中去除汞提供了一种经济高效且环保的方法。比堇青石-hs 243 g / m3高约14倍。Cu-hs优异的Hg0吸附性能归因于Cu2Se中硒对汞的高亲和力,Cu2Se的均匀分布以及Cu泡沫基材的优异流体特性。通过HCl溶液浸出和随后的再硒化可以有效地恢复废Cu-hs的吸附性能。可循环利用的铜-硫化氢的使用为从工业烟气中去除汞提供了一种经济高效且环保的方法。比堇青石-hs 243 g / m3高约14倍。Cu-hs优异的Hg0吸附性能归因于Cu2Se中硒对汞的高亲和力,Cu2Se的均匀分布以及Cu泡沫基材的优异流体特性。通过HCl溶液浸出和随后的再硒化可以有效地恢复废Cu-hs的吸附性能。可循环利用的铜-硫化氢的使用为从工业烟气中去除汞提供了一种经济高效且环保的方法。通过HCl溶液浸出和随后的再硒化可以有效地恢复废Cu-hs的吸附性能。可循环利用的铜-硫化氢的使用为从工业烟气中去除汞提供了一种经济高效且环保的方法。通过HCl溶液浸出和随后的再硒化可以有效地恢复废Cu-hs的吸附性能。可循环利用的铜-硫化氢的使用为从工业烟气中去除汞提供了一种经济高效且环保的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号