当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human Plasma Protein Corona of Aβ Amyloid and Its Impact on Islet Amyloid Polypeptide Cross-Seeding.
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-01-21 , DOI: 10.1021/acs.biomac.9b01650 Aparna Nandakumar 1 , Yanting Xing 2 , Ritchlynn R Aranha 3 , Ava Faridi 1 , Aleksandr Kakinen 1 , Ibrahim Javed 1 , Kairi Koppel 1 , Emily H Pilkington 1 , Anthony Wayne Purcell 3 , Thomas P Davis 1, 4 , Pouya Faridi 3 , Feng Ding 2 , Pu Chun Ke 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-01-21 , DOI: 10.1021/acs.biomac.9b01650 Aparna Nandakumar 1 , Yanting Xing 2 , Ritchlynn R Aranha 3 , Ava Faridi 1 , Aleksandr Kakinen 1 , Ibrahim Javed 1 , Kairi Koppel 1 , Emily H Pilkington 1 , Anthony Wayne Purcell 3 , Thomas P Davis 1, 4 , Pouya Faridi 3 , Feng Ding 2 , Pu Chun Ke 1
Affiliation
|
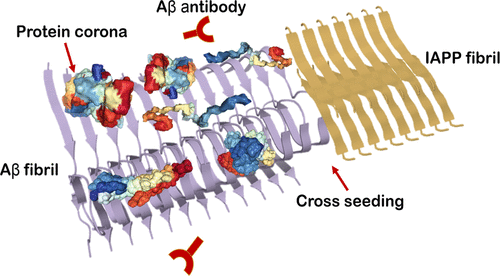
Alzheimer's disease (AD) is the most severe form of neurological disorder, characterized by the presence of extracellular amyloid-β (Aβ) plaques and intracellular tau tangles. For decades, therapeutic strategies against the pathological symptoms of AD have often relied on the delivery of monoclonal antibodies to target specifically Aβ amyloid or oligomers, largely to no avail. Aβ can be traced in the brain as well as in cerebrospinal fluid and the circulation, giving rise to abundant opportunities to interact with their environmental proteins. Using liquid chromatography tandem-mass spectrometry, here we identified for the first time the protein coronae of the two major amyloid forms of Aβ-Aβ1-42 and Aβ1-40-exposed to human blood plasma. Out of the proteins identified in all groups, 58 proteins were unique to the Aβ1-42 samples and 31 proteins unique to the Aβ1-40 samples. Both fibrillar coronae consisted of proteins significant in complement activation, inflammation, and protein metabolic pathways involved in the pathology of AD. Structure-wise, the coronal proteins often possessed multidomains of high flexibility to maximize their association with the amyloid fibrils. The protein corona hindered recognition of Aβ1-42 fibrils by their structurally specific antibodies and accelerated the aggregation but not the β-cell toxicity of human islet amyloid polypeptide, the peptide associated with type 2 diabetes. This study highlights the importance of understanding the structural, functional, and pathological implications of the amyloid protein corona for the development of therapeutics against AD and a range of amyloid diseases.
中文翻译:

Aβ 淀粉样蛋白的人血浆蛋白冠及其对胰岛淀粉样蛋白多肽交叉播种的影响。
阿尔茨海默病 (AD) 是最严重的神经系统疾病,其特征是存在细胞外淀粉样蛋白 - β (Aβ) 斑块和细胞内 tau 缠结。几十年来,针对 AD 病理症状的治疗策略通常依赖于递送单克隆抗体来特异性靶向 Aβ 淀粉样蛋白或寡聚体,但基本上没有效果。 Aβ 可以在大脑、脑脊液和循环中追踪到,从而提供了大量与其环境蛋白质相互作用的机会。使用液相色谱串联质谱法,我们首次鉴定了暴露于人血浆的两种主要淀粉样蛋白形式 Aβ-Aβ1-42 和 Aβ1-40-的蛋白冠。在所有组中鉴定的蛋白质中,有 58 种蛋白质是 Aβ1-42 样品特有的,31 种蛋白质是 Aβ1-40 样品特有的。两种纤维状冠均由对补体激活、炎症和 AD 病理学相关的蛋白质代谢途径具有重要意义的蛋白质组成。在结构方面,冠状蛋白通常具有高灵活性的多结构域,以最大限度地提高它们与淀粉样原纤维的关联。蛋白冠阻碍了结构特异性抗体对 Aβ1-42 原纤维的识别,并加速了人胰岛淀粉样多肽(与 2 型糖尿病相关的肽)的聚集,但没有加速其 β 细胞毒性。这项研究强调了了解淀粉样蛋白冠的结构、功能和病理学影响对于开发针对 AD 和一系列淀粉样蛋白疾病的疗法的重要性。
更新日期:2020-01-22
中文翻译:

Aβ 淀粉样蛋白的人血浆蛋白冠及其对胰岛淀粉样蛋白多肽交叉播种的影响。
阿尔茨海默病 (AD) 是最严重的神经系统疾病,其特征是存在细胞外淀粉样蛋白 - β (Aβ) 斑块和细胞内 tau 缠结。几十年来,针对 AD 病理症状的治疗策略通常依赖于递送单克隆抗体来特异性靶向 Aβ 淀粉样蛋白或寡聚体,但基本上没有效果。 Aβ 可以在大脑、脑脊液和循环中追踪到,从而提供了大量与其环境蛋白质相互作用的机会。使用液相色谱串联质谱法,我们首次鉴定了暴露于人血浆的两种主要淀粉样蛋白形式 Aβ-Aβ1-42 和 Aβ1-40-的蛋白冠。在所有组中鉴定的蛋白质中,有 58 种蛋白质是 Aβ1-42 样品特有的,31 种蛋白质是 Aβ1-40 样品特有的。两种纤维状冠均由对补体激活、炎症和 AD 病理学相关的蛋白质代谢途径具有重要意义的蛋白质组成。在结构方面,冠状蛋白通常具有高灵活性的多结构域,以最大限度地提高它们与淀粉样原纤维的关联。蛋白冠阻碍了结构特异性抗体对 Aβ1-42 原纤维的识别,并加速了人胰岛淀粉样多肽(与 2 型糖尿病相关的肽)的聚集,但没有加速其 β 细胞毒性。这项研究强调了了解淀粉样蛋白冠的结构、功能和病理学影响对于开发针对 AD 和一系列淀粉样蛋白疾病的疗法的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号