当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Highly Functionalized 12-Membered Trifluoromethyl Heterocycles via a Nondecarboxylative Pd-Catalyzed [6 + 6] Annulation
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-07 , DOI: 10.1021/acscatal.9b05377 Hiroto Uno 1 , Takanori Imai 1 , Kyosuke Harada 1 , Norio Shibata 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-07 , DOI: 10.1021/acscatal.9b05377 Hiroto Uno 1 , Takanori Imai 1 , Kyosuke Harada 1 , Norio Shibata 1, 2
Affiliation
|
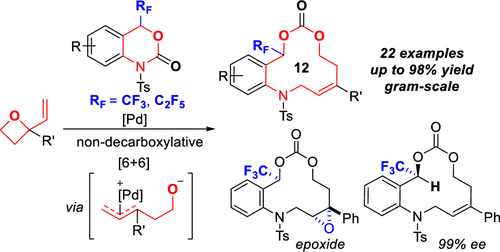
An efficient method for the construction of 12-membered heterocycles with a trifluoromethyl group (3) was achieved via a nondecarboxylative Pd-catalyzed [6 + 6] annulation of six-membered trifluoromethyl benzo[d][1,3]oxazinones (1) with four-membered vinyl oxetanes (2). A variety of relatively large heterocycles 3 featuring a stereogenic trifluoromethylated carbon center, an amino group, as well as an alkenyl and a carbonate moiety were obtained. An X-ray crystallographic analysis revealed a unique 3D architecture of the products that affords an attractive chemical space. Whereas the method can be applicable for nonfluorinated substrates, the trifluoromethyl group plays a vital role in this unexpected Pd-catalyzed nondecarboxylative ring expansion.
中文翻译:

通过非脱羧的Pd催化的[6 + 6]环合成高功能化的12元三氟甲基杂环
为12元与三氟甲基(杂环构造一个有效的方法3经由六元三氟甲基苯并[nondecarboxylative Pd催化[6 + 6]环达到)d ] [1,3] oxazinones(1)与四元乙烯基氧杂环丁烷(2)。各种相对较大的杂环3获得具有立体异构的三氟甲基化碳中心的特征,即氨基,烯基和碳酸酯部分。X射线晶体学分析揭示了该产品独特的3D结构,提供了诱人的化学空间。尽管该方法可用于非氟化底物,但是三氟甲基在这种意外的Pd催化的非脱羧环膨胀中起着至关重要的作用。
更新日期:2020-01-07
中文翻译:

通过非脱羧的Pd催化的[6 + 6]环合成高功能化的12元三氟甲基杂环
为12元与三氟甲基(杂环构造一个有效的方法3经由六元三氟甲基苯并[nondecarboxylative Pd催化[6 + 6]环达到)d ] [1,3] oxazinones(1)与四元乙烯基氧杂环丁烷(2)。各种相对较大的杂环3获得具有立体异构的三氟甲基化碳中心的特征,即氨基,烯基和碳酸酯部分。X射线晶体学分析揭示了该产品独特的3D结构,提供了诱人的化学空间。尽管该方法可用于非氟化底物,但是三氟甲基在这种意外的Pd催化的非脱羧环膨胀中起着至关重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号