当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pt-Assisted Carbon Remediation of Mo2C Materials for CO Disproportionation
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-16 , DOI: 10.1021/acscatal.9b05225 Zongtang Fang 1 , Lu-Cun Wang 1 , Yixiao Wang 1 , Ember Sikorski 2, 3 , Shuai Tan 3, 4 , Katie Dongmei Li-Oakey 3, 4 , Lan Li 2, 3 , Gregory Yablonsky 5 , David A. Dixon 6 , Rebecca Fushimi 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-16 , DOI: 10.1021/acscatal.9b05225 Zongtang Fang 1 , Lu-Cun Wang 1 , Yixiao Wang 1 , Ember Sikorski 2, 3 , Shuai Tan 3, 4 , Katie Dongmei Li-Oakey 3, 4 , Lan Li 2, 3 , Gregory Yablonsky 5 , David A. Dixon 6 , Rebecca Fushimi 1
Affiliation
|
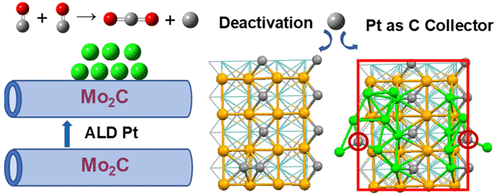
Using the CO disproportionation (Boudouard) reaction as a probe reaction, an in-depth analysis of temperature-programmed pulse response data shows that the addition of Pt to Mo2C mitigates deactivation of Mo active sites by acting as a carbon collector. CO2 production on Mo2C and Pt/Mo2C materials is dependent on both the activation energy and the CO surface concentration. Detailed plane-wave density functional theory calculations of the CO adsorption and disproportion reactions on Mo2C-supported Pt nanoparticles (NPs) are reported. The Mo2C was modeled by the β-Mo2C (100) surface, and the Pt/Mo2C interface was modeled by the addition of 12 Pt atoms to the Mo2C (100) surface ([email protected]2C). The potential energy surfaces of the Boudouard reaction were calculated on pure Mo2C, [email protected]2C, and Pt (111) surfaces. CO dissociation readily occurs on the Mo2C (100) surface, but not on the Pt (111) surface, with the former being exothermic and the latter being endothermic. At the Pt/Mo2C interface, CO dissociation is still exothermic, but with a larger energy barrier. The Boudouard reaction takes place on the Mo2C region, where CO2 is formed from a surface O atom dissociated from one CO molecule in reaction with another CO molecule, leaving one C atom on the surface. C adsorption is preferential on the Pt site in comparison to the Mo site. The supported Pt domains can collect the remaining C atoms, facilitating further CO2 formation on the active Mo sites. A Bader charge analysis shows that the surface metal–carbon bond is a mixture of covalent and ionic bonds, whereas the surface metal–oxygen bond is ionic. Electron localization function (ELF) and partial charge density calculations agree well with the Bader charge analysis. These computational results are consistent with experimental observations of the interaction of CO with Mo2C nanotube supported Pt domains in the transient regime under far from equilibrium conditions. The Boudouard reaction is an important side reaction, and the unexpected role found for Pt as a carbon collector, with Mo serving as a disproportionation site, provides a unique vantage point for understanding carbon and coke formation on catalytic materials.
中文翻译:

Mo 2 C材料的Pt辅助碳修复以实现CO歧化
使用CO歧化(Boudouard)反应作为探针反应,对温度编程的脉冲响应数据的深入分析表明,向Mo 2 C添加Pt可以通过充当碳收集器来缓解Mo活性位的失活。Mo 2 C和Pt / Mo 2 C材料上的CO 2产生取决于活化能和CO表面浓度。报道了在Mo 2 C负载的Pt纳米颗粒(NPs)上CO吸附和歧化反应的详细平面波密度泛函理论计算。在Mo 2 C是通过在β-沫建模2 C(100)面,并且Pt /沫2通过在Mo 2 C(100)表面([受电子邮件保护] 2 C)上添加12个Pt原子来模拟C界面。在纯Mo 2 C,[受电子邮件保护] 2 C和Pt(111)表面上计算了Boudouard反应的势能面。CO的解离很容易在Mo 2 C(100)表面发生,而在Pt(111)表面不发生,前者是放热的,后者是吸热的。在Pt / Mo 2 C界面处,CO的解离仍然是放热的,但具有较大的能垒。Boudouard反应在Mo 2 C区域发生,其中CO 2由一个与一个CO分子解离的表面O原子与另一个CO分子反应而形成,在表面上留下一个C原子。与Mo位相比,Pt位上的C吸附优先。负载的Pt域可以收集剩余的C原子,从而有助于在活性Mo位上进一步形成CO 2。Bader电荷分析表明,表面金属-碳键是共价键和离子键的混合物,而表面金属-氧键是离子键。电子定位函数(ELF)和部分电荷密度计算与Bader电荷分析非常吻合。这些计算结果与CO与Mo 2相互作用的实验观察结果一致C纳米管在远离平衡条件的瞬态中支持Pt域。Boudouard反应是重要的副反应,Pt作为碳捕集剂,Mo作为歧化位点,出乎意料地发挥了作用,为理解催化材料上的碳和焦炭形成提供了独特的优势。
更新日期:2020-01-16
中文翻译:

Mo 2 C材料的Pt辅助碳修复以实现CO歧化
使用CO歧化(Boudouard)反应作为探针反应,对温度编程的脉冲响应数据的深入分析表明,向Mo 2 C添加Pt可以通过充当碳收集器来缓解Mo活性位的失活。Mo 2 C和Pt / Mo 2 C材料上的CO 2产生取决于活化能和CO表面浓度。报道了在Mo 2 C负载的Pt纳米颗粒(NPs)上CO吸附和歧化反应的详细平面波密度泛函理论计算。在Mo 2 C是通过在β-沫建模2 C(100)面,并且Pt /沫2通过在Mo 2 C(100)表面([受电子邮件保护] 2 C)上添加12个Pt原子来模拟C界面。在纯Mo 2 C,[受电子邮件保护] 2 C和Pt(111)表面上计算了Boudouard反应的势能面。CO的解离很容易在Mo 2 C(100)表面发生,而在Pt(111)表面不发生,前者是放热的,后者是吸热的。在Pt / Mo 2 C界面处,CO的解离仍然是放热的,但具有较大的能垒。Boudouard反应在Mo 2 C区域发生,其中CO 2由一个与一个CO分子解离的表面O原子与另一个CO分子反应而形成,在表面上留下一个C原子。与Mo位相比,Pt位上的C吸附优先。负载的Pt域可以收集剩余的C原子,从而有助于在活性Mo位上进一步形成CO 2。Bader电荷分析表明,表面金属-碳键是共价键和离子键的混合物,而表面金属-氧键是离子键。电子定位函数(ELF)和部分电荷密度计算与Bader电荷分析非常吻合。这些计算结果与CO与Mo 2相互作用的实验观察结果一致C纳米管在远离平衡条件的瞬态中支持Pt域。Boudouard反应是重要的副反应,Pt作为碳捕集剂,Mo作为歧化位点,出乎意料地发挥了作用,为理解催化材料上的碳和焦炭形成提供了独特的优势。










































 京公网安备 11010802027423号
京公网安备 11010802027423号