当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Celastrol-Loaded Galactosylated Liposomes Effectively Inhibit AKT/c-Met-Triggered Rapid Hepatocarcinogenesis in Mice.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-02-03 , DOI: 10.1021/acs.molpharmaceut.9b00428 Xinyan Chen 1 , Xianxian Hu 1 , Junjie Hu 1 , Zhenpeng Qiu 1 , Ming Yuan 1 , Guohua Zheng 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-02-03 , DOI: 10.1021/acs.molpharmaceut.9b00428 Xinyan Chen 1 , Xianxian Hu 1 , Junjie Hu 1 , Zhenpeng Qiu 1 , Ming Yuan 1 , Guohua Zheng 2
Affiliation
|
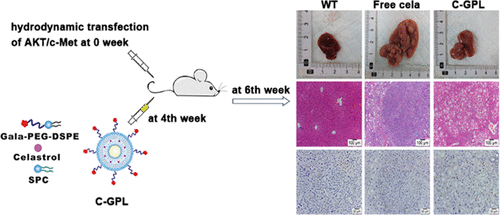
Our previous study proved that celastrol was a potential candidate for hepatocellular carcinoma (HCC) therapy. However, poor water solubility and toxic side effects may restrict its clinical application. To overcome these shortcomings and optimize its antitumor efficacy, we developed galactosylated liposomes using galactose-modified 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol) to deliver celastrol (C-GPL). C-GPL improved the water solubility of celastrol and exhibited high encapsulation efficiency, good stability in serum, and slow drug release profile. In vitro studies showed that C-GPL increased the cellular uptake of celastrol through receptor-mediated endocytosis, thereby enhancing celastrol cytotoxicity and cancer cell apoptosis. Particularly, in vivo antitumor activity of C-GPL was assessed in rapid HCC mouse models established via hydrodynamic transfection of the activated forms of AKT and c-Met. Compared to free celastrol, C-GPL significantly prevented liver weight gain, decreased liver damage biomarkers (glutamic-oxalacetic transaminase and alanine aminotransferase) and HCC marker (alpha-fetoprotein), and led to tumor disappearance on the liver surface. The improved therapeutic effect of C-GPL may be attributed to suppression of AKT activation, induction of apoptosis, and retardation of cell proliferation. Importantly, C-GPL exerted low toxicity to normal tissues without causing severe weight loss in mice. Taken together, C-GPL may become a promising drug delivery system for HCC treatment.
中文翻译:

Celastrol负载的半乳糖基化脂质体有效抑制小鼠AKT / c-Met触发的快速肝癌发生。
我们之前的研究证明,Celastrol是肝细胞癌(HCC)治疗的潜在候选人。但是,水溶性差和毒副作用可能会限制其临床应用。为克服这些缺点并优化其抗肿瘤功效,我们使用半乳糖修饰的1,2-二硬脂酰基-sn-甘油-3-磷酸乙醇胺-聚乙二醇来开发半乳糖基化脂质体,以递送Celastrol(C-GPL)。C-GPL改善了Celastrol的水溶性,并显示出高包封效率,在血清中良好的稳定性和缓慢的药物释放曲线。体外研究表明,C-GPL通过受体介导的内吞作用增加了对Celastrol的细胞摄取,从而增强了Celastrol的细胞毒性和癌细胞凋亡。尤其,在通过激活形式的AKT和c-Met的流体动力学转染建立的快速HCC小鼠模型中评估了C-GPL的体内抗肿瘤活性。与游离的Celastrol相比,C-GPL显着阻止了肝脏增重,降低了肝损伤生物标志物(谷氨酸-草酰乙酸转氨酶和丙氨酸转氨酶)和HCC标志物(甲胎蛋白),并导致肝脏表面肿瘤消失。C-GPL的治疗效果改善可能归因于AKT激活的抑制,细胞凋亡的诱导和细胞增殖的延迟。重要的是,C-GPL对正常组织的毒性低,而不会引起小鼠体重的严重减轻。综上所述,C-GPL可能成为有希望的用于HCC治疗的药物递送系统。与游离的Celastrol相比,C-GPL显着阻止了肝脏增重,降低了肝损伤生物标志物(谷氨酸-草酰乙酸转氨酶和丙氨酸转氨酶)和HCC标志物(甲胎蛋白),并导致肝脏表面肿瘤消失。C-GPL的治疗效果改善可能归因于AKT激活的抑制,细胞凋亡的诱导和细胞增殖的延迟。重要的是,C-GPL对正常组织的毒性低,而不会引起小鼠体重的严重减轻。综上所述,C-GPL可能成为有希望的用于HCC治疗的药物递送系统。与游离的Celastrol相比,C-GPL显着阻止了肝脏增重,降低了肝损伤生物标志物(谷氨酸-草酰乙酸转氨酶和丙氨酸转氨酶)和HCC标志物(甲胎蛋白),并导致肝脏表面肿瘤消失。C-GPL的治疗效果改善可能归因于AKT激活的抑制,细胞凋亡的诱导和细胞增殖的延迟。重要的是,C-GPL对正常组织的毒性低,而不会引起小鼠体重的严重减轻。综上所述,C-GPL可能成为有希望的用于HCC治疗的药物递送系统。C-GPL的治疗效果改善可能归因于AKT激活的抑制,细胞凋亡的诱导和细胞增殖的延迟。重要的是,C-GPL对正常组织的毒性低,而不会引起小鼠体重的严重减轻。综上所述,C-GPL可能成为有希望的用于HCC治疗的药物递送系统。C-GPL的治疗效果改善可能归因于AKT激活的抑制,细胞凋亡的诱导和细胞增殖的延迟。重要的是,C-GPL对正常组织的毒性低,而不会引起小鼠体重的严重减轻。综上所述,C-GPL可能成为用于HCC治疗的有希望的药物输送系统。
更新日期:2020-02-04
中文翻译:

Celastrol负载的半乳糖基化脂质体有效抑制小鼠AKT / c-Met触发的快速肝癌发生。
我们之前的研究证明,Celastrol是肝细胞癌(HCC)治疗的潜在候选人。但是,水溶性差和毒副作用可能会限制其临床应用。为克服这些缺点并优化其抗肿瘤功效,我们使用半乳糖修饰的1,2-二硬脂酰基-sn-甘油-3-磷酸乙醇胺-聚乙二醇来开发半乳糖基化脂质体,以递送Celastrol(C-GPL)。C-GPL改善了Celastrol的水溶性,并显示出高包封效率,在血清中良好的稳定性和缓慢的药物释放曲线。体外研究表明,C-GPL通过受体介导的内吞作用增加了对Celastrol的细胞摄取,从而增强了Celastrol的细胞毒性和癌细胞凋亡。尤其,在通过激活形式的AKT和c-Met的流体动力学转染建立的快速HCC小鼠模型中评估了C-GPL的体内抗肿瘤活性。与游离的Celastrol相比,C-GPL显着阻止了肝脏增重,降低了肝损伤生物标志物(谷氨酸-草酰乙酸转氨酶和丙氨酸转氨酶)和HCC标志物(甲胎蛋白),并导致肝脏表面肿瘤消失。C-GPL的治疗效果改善可能归因于AKT激活的抑制,细胞凋亡的诱导和细胞增殖的延迟。重要的是,C-GPL对正常组织的毒性低,而不会引起小鼠体重的严重减轻。综上所述,C-GPL可能成为有希望的用于HCC治疗的药物递送系统。与游离的Celastrol相比,C-GPL显着阻止了肝脏增重,降低了肝损伤生物标志物(谷氨酸-草酰乙酸转氨酶和丙氨酸转氨酶)和HCC标志物(甲胎蛋白),并导致肝脏表面肿瘤消失。C-GPL的治疗效果改善可能归因于AKT激活的抑制,细胞凋亡的诱导和细胞增殖的延迟。重要的是,C-GPL对正常组织的毒性低,而不会引起小鼠体重的严重减轻。综上所述,C-GPL可能成为有希望的用于HCC治疗的药物递送系统。与游离的Celastrol相比,C-GPL显着阻止了肝脏增重,降低了肝损伤生物标志物(谷氨酸-草酰乙酸转氨酶和丙氨酸转氨酶)和HCC标志物(甲胎蛋白),并导致肝脏表面肿瘤消失。C-GPL的治疗效果改善可能归因于AKT激活的抑制,细胞凋亡的诱导和细胞增殖的延迟。重要的是,C-GPL对正常组织的毒性低,而不会引起小鼠体重的严重减轻。综上所述,C-GPL可能成为有希望的用于HCC治疗的药物递送系统。C-GPL的治疗效果改善可能归因于AKT激活的抑制,细胞凋亡的诱导和细胞增殖的延迟。重要的是,C-GPL对正常组织的毒性低,而不会引起小鼠体重的严重减轻。综上所述,C-GPL可能成为有希望的用于HCC治疗的药物递送系统。C-GPL的治疗效果改善可能归因于AKT激活的抑制,细胞凋亡的诱导和细胞增殖的延迟。重要的是,C-GPL对正常组织的毒性低,而不会引起小鼠体重的严重减轻。综上所述,C-GPL可能成为用于HCC治疗的有希望的药物输送系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号