当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ent-Labdane and ent-kaurane diterpenoids from Chelonopsis odontochila with α-glucosidase inhibitory activity.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.bioorg.2020.103571 Zhen-Tao Deng 1 , Ji-Jun Chen 2 , Chang-An Geng 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.bioorg.2020.103571 Zhen-Tao Deng 1 , Ji-Jun Chen 2 , Chang-An Geng 1
Affiliation
|
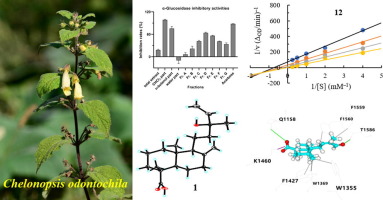
Eleven new ent-labdane diterpenoids, cheodontoins A-K (1-11), and thirteen known diterpenoids involving two ent-labdanes (12-13) and eleven ent-kauranes (14-24), were isolated from the active part of Chelonopsis odontochila (Lamiaceae) under the guidance of bioassay. The structures of cheodontoins A-K (1-11) were elucidated by extensive HRESIMS, 1D and 2D NMR, [α]D and ECD experiments, X-ray diffraction and quantum calculation. Interestingly, five nor-ent-labdanes (9-13) were obtained from this genus for the first time. One ent-labdane diterpenoid (12) and four ent-kaurane diterpenoids (16, 19, 23, and 24) showed α-glucosidase inhibitory activity with IC50 values of 326.5 ± 3.5, 599.1 ± 13.8, 620.1 ± 16.1, 185.0 ± 4.2, and 190.7 ± 11.6 μM, respectively. Compounds 12 and 16 were α-glucosidase mixed-type inhibitors with Ki values of 334.1 and 589.2 μM according to the enzyme kinetics using Lineweaver-Burk and Dixon plots. Docking study manifested that compounds 12 and 23 well located in the catalytic pocket of α-glucosidase by hydrophobic effects with Trp1355, Trp1369, Phe1427, Phe1559, and Phe1560 residues. This study provides new insights for the antidiabetic effects of C. odontochila with ent-labdane and ent-kaurane diterpenoids as the active constituents.
中文翻译:

齿形龙(Chelonopsis odontochila)的对-拉丹烷和对-月桂烷双萜类化合物具有α-葡萄糖苷酶抑制活性。
从猪牙白屈病(Chelonopsis odontochila)的活性部分中分离出了11种新的拉丹烷二萜类化合物,天青蛋白AK(1-11)和13种已知的涉及两对拉丹类化合物(12-13)和11种高伯灵(14-24)的二萜类化合物(唇形科)在生物测定的指导下。通过广泛的HRESIMS,1D和2D NMR,[α] D和ECD实验,X射线衍射和量子计算,阐明了化学牙本质素AK(1-11)的结构。有趣的是,首次从该属中获得了5个nor-ent-labdanes(9-13)。1个拉丹烷二萜类化合物(12)和4个月桂烷双萜类化合物(16、19、23和24)显示出α-葡萄糖苷酶抑制活性,IC50值为326.5±3.5、599.1±13.8、620.1±16.1、185.0±4.2,和190.7±11.6μM。化合物12和16是α-葡萄糖苷酶混合型抑制剂,Ki值为334.1和589。根据酶动力学,使用Lineweaver-Burk和Dixon图绘制2μM。对接研究表明,化合物12和23通过Trp1355,Trp1369,Phe1427,Phe1559和Phe1560残基的疏水作用很好地位于α-葡萄糖苷酶的催化口袋中。这项研究为以恩达巴丹和恩-金刚烷二萜类化合物为有效成分的C. odontochila的抗糖尿病作用提供了新见识。
更新日期:2020-01-07
中文翻译:

齿形龙(Chelonopsis odontochila)的对-拉丹烷和对-月桂烷双萜类化合物具有α-葡萄糖苷酶抑制活性。
从猪牙白屈病(Chelonopsis odontochila)的活性部分中分离出了11种新的拉丹烷二萜类化合物,天青蛋白AK(1-11)和13种已知的涉及两对拉丹类化合物(12-13)和11种高伯灵(14-24)的二萜类化合物(唇形科)在生物测定的指导下。通过广泛的HRESIMS,1D和2D NMR,[α] D和ECD实验,X射线衍射和量子计算,阐明了化学牙本质素AK(1-11)的结构。有趣的是,首次从该属中获得了5个nor-ent-labdanes(9-13)。1个拉丹烷二萜类化合物(12)和4个月桂烷双萜类化合物(16、19、23和24)显示出α-葡萄糖苷酶抑制活性,IC50值为326.5±3.5、599.1±13.8、620.1±16.1、185.0±4.2,和190.7±11.6μM。化合物12和16是α-葡萄糖苷酶混合型抑制剂,Ki值为334.1和589。根据酶动力学,使用Lineweaver-Burk和Dixon图绘制2μM。对接研究表明,化合物12和23通过Trp1355,Trp1369,Phe1427,Phe1559和Phe1560残基的疏水作用很好地位于α-葡萄糖苷酶的催化口袋中。这项研究为以恩达巴丹和恩-金刚烷二萜类化合物为有效成分的C. odontochila的抗糖尿病作用提供了新见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号