当前位置:
X-MOL 学术
›
Cell. Signal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
KRIT1 loss-mediated upregulation of NOX1 in stromal cells promotes paracrine pro-angiogenic responses.
Cellular Signalling ( IF 4.4 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.cellsig.2020.109527 Federica Finetti 1 , Irene Schiavo 1 , Jasmine Ercoli 1 , Alessia Zotta 2 , Enrica Boda 3 , Saverio Francesco Retta 2 , Lorenza Trabalzini 1
Cellular Signalling ( IF 4.4 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.cellsig.2020.109527 Federica Finetti 1 , Irene Schiavo 1 , Jasmine Ercoli 1 , Alessia Zotta 2 , Enrica Boda 3 , Saverio Francesco Retta 2 , Lorenza Trabalzini 1
Affiliation
|
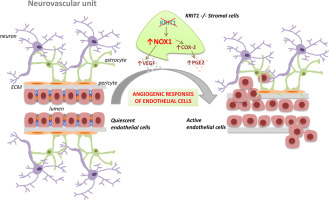
Cerebral cavernous malformation (CCM) is a cerebrovascular disorder of proven genetic origin characterized by abnormally dilated and leaky capillaries occurring mainly in the central nervous system, with a prevalence of 0.3-0.5% in the general population. Genetic studies have identified causative mutations in three genes, CCM1/KRIT1, CCM2 and CCM3, which are involved in the maintenance of vascular homeostasis. However, distinct studies in animal models have clearly shown that CCM gene mutations alone are not sufficient to cause CCM disease, but require additional contributing factors, including stochastic events of increased oxidative stress and inflammation. Consistently, previous studies have shown that up-regulation of NADPH oxidase-mediated production of reactive oxygen species (ROS) in KRIT1 deficient endothelium contributes to the loss of microvessel barrier function. In this study, we demonstrate that KRIT1 loss-of-function in stromal cells, such as fibroblasts, causes the up-regulation of NADPH oxidase isoform 1 (NOX1) and the activation of inflammatory pathways, which in turn promote an enhanced production of proangiogenic factors, including vascular endothelial growth factor (VEGF) and prostaglandin E2 (PGE2). Furthermore and importantly, we show that conditioned media from KRIT1 null fibroblasts induce proliferation, migration, matrix metalloproteinase 2 (MMP2) activation and VE-cadherin redistribution in wild type human endothelial cells. Taken together, our results demonstrate that KRIT1 loss-of-function in stromal cells affects the surrounding microenvironment through a NOX1-mediated induction and release of angiogenic factors that are able to promote paracrine proangiogenic responses in human endothelial cells, thus pointing to a novel role for endothelial cell-nonautonomous effects of KRIT1 mutations in CCM pathogenesis, and opening new perspectives for disease prevention and treatment.
中文翻译:

基质细胞中KRIT1损失介导的NOX1上调促进旁分泌促血管生成反应。
脑海绵状畸形(CCM)是一种脑血管疾病,已证明具有遗传起源,其特征是主要在中枢神经系统中发生异常扩张和渗漏的毛细血管,在一般人群中患病率为0.3-0.5%。遗传研究确定了三个基因CCM1 / KRIT1,CCM2和CCM3的致病突变,这些基因与维持血管稳态有关。但是,在动物模型中进行的不同研究清楚地表明,仅CCM基因突变不足以引起CCM疾病,而是需要其他贡献因素,包括氧化应激和炎症增加的随机事件。一致地,先前的研究表明,在KRIT1缺陷型内皮细胞中,NADPH氧化酶介导的活性氧(ROS)产生的上调会导致微血管屏障功能的丧失。在这项研究中,我们证明了基质细胞(如成纤维细胞)中的KRIT1功能丧失导致NADPH氧化酶同工型1(NOX1)的上调和炎症途径的激活,进而促进促血管生成的产生因子,包括血管内皮生长因子(VEGF)和前列腺素E2(PGE2)。此外,重要的是,我们显示来自KRIT1无效成纤维细胞的条件培养基在野生型人内皮细胞中诱导增殖,迁移,基质金属蛋白酶2(MMP2)激活和VE-钙黏着蛋白再分布。在一起
更新日期:2020-01-07
中文翻译:

基质细胞中KRIT1损失介导的NOX1上调促进旁分泌促血管生成反应。
脑海绵状畸形(CCM)是一种脑血管疾病,已证明具有遗传起源,其特征是主要在中枢神经系统中发生异常扩张和渗漏的毛细血管,在一般人群中患病率为0.3-0.5%。遗传研究确定了三个基因CCM1 / KRIT1,CCM2和CCM3的致病突变,这些基因与维持血管稳态有关。但是,在动物模型中进行的不同研究清楚地表明,仅CCM基因突变不足以引起CCM疾病,而是需要其他贡献因素,包括氧化应激和炎症增加的随机事件。一致地,先前的研究表明,在KRIT1缺陷型内皮细胞中,NADPH氧化酶介导的活性氧(ROS)产生的上调会导致微血管屏障功能的丧失。在这项研究中,我们证明了基质细胞(如成纤维细胞)中的KRIT1功能丧失导致NADPH氧化酶同工型1(NOX1)的上调和炎症途径的激活,进而促进促血管生成的产生因子,包括血管内皮生长因子(VEGF)和前列腺素E2(PGE2)。此外,重要的是,我们显示来自KRIT1无效成纤维细胞的条件培养基在野生型人内皮细胞中诱导增殖,迁移,基质金属蛋白酶2(MMP2)激活和VE-钙黏着蛋白再分布。在一起











































 京公网安备 11010802027423号
京公网安备 11010802027423号