当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vapor Pressure Measurement of Ternary Systems LiBr + [Emim]I + H2O and LiBr + [Dmim]I + H2O
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jced.9b00614 Yongmei Xuan 1 , Kaile Fang 1 , Bosheng Duan 1 , Neng Gao 1 , Guangming Chen 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jced.9b00614 Yongmei Xuan 1 , Kaile Fang 1 , Bosheng Duan 1 , Neng Gao 1 , Guangming Chen 1
Affiliation
|
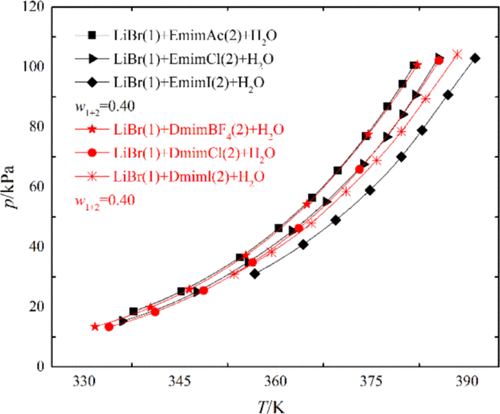
Ionic liquids (ILs), 1-ethyl-3-methylimidazolium iodine [Emim]I and 1,3-dimethylimidazolium iodine [Dmim]I, were proposed as a novel potential solvent in traditional bromide aqueous solution (H2O + LiBr) in absorption refrigeration cycles. The vapor pressure data of two ternary systems H2O + LiBr + [Emim]I (mass ratio LiBr/[Emim]I = 4) at temperatures from 350.78 to 414.19 K and H2O + LiBr + [Dmim]I (mass ratio LiBr/[Dmim]I = 2) at temperatures from 350.69 to 409.63 K were reported by the boiling point method. The absorbent mass fractions are from 0.30 to 0.57 and 0.30 to 0.60, respectively. The measured data were correlated by using the Antoine-type equation, and the average absolute relative deviation (AARD %) between measured data and regression data was 0.54 and 0.39%. Compared with the vapor pressures of IL mixtures with the same organic cation, the vapor pressure order is LiBr + [Emim]I + H2O < LiBr + [Emim]Cl + H2O < LiBr + [Emim]Ac + H2O and LiBr + [Dmim]I + H2O < LiBr + [Dmim]Cl + H2O < LiBr + [Dmim]BF4 + H2O. Under experimental conditions, the hydrophilic properties of the iodine anion (I–) are better than those of the chlorine anion (Cl–), acetate anion (Ac–), and tetrafluoroborate anion (BF4–). Lower vapor pressure means stronger moisture absorption property. The proposed ternary mixtures have lower vapor pressures and can improve the absorption effect of the absorption system.
中文翻译:

三元体系LiBr + [Emim] I + H 2 O和LiBr + [Dmim] I + H 2 O的蒸气压测量
在传统的溴化物水溶液(H 2 O + LiBr)中,提出了一种离子液体(IL)1-乙基-3-甲基咪唑碘[Emim] I和1,3-二甲基咪唑碘[Dmim] I。吸收式制冷循环。在350.78至414.19 K和H 2的温度下,两个三元系统H 2 O + LiBr + [Emim] I(质量比LiBr / [Emim] I = 4)的蒸气压数据通过沸点法报道了在350.69至409.63K的温度下的O + LiBr + [Dmim] I(质量比LiBr / [Dmim] I = 2)。吸收剂质量分数分别为0.30至0.57和0.30至0.60。使用Antoine型方程式对测量数据进行关联,测量数据与回归数据之间的平均绝对相对偏差(AARD%)为0.54和0.39%。与具有相同有机阳离子的IL混合物的蒸气压相比,蒸气压顺序为LiBr + [Emim] I + H 2 O <LiBr + [Emim] Cl + H 2 O <LiBr + [Emim] Ac + H 2 O和LiBr + [Dmim] I + H 2 O <LiBr + [Dmim] Cl + H 2 O <LiBr + [Dmim] BF 4 + H 2O.在实验条件下,碘阴离子(I的亲水性- )比那些氯阴离子(CL的更好- ),乙酸根阴离子(AC - ),和四氟硼酸阴离子(BF 4 - )。较低的蒸气压意味着更强的吸湿性能。所提出的三元混合物具有较低的蒸气压并且可以改善吸收系统的吸收效果。
更新日期:2020-01-07
中文翻译:

三元体系LiBr + [Emim] I + H 2 O和LiBr + [Dmim] I + H 2 O的蒸气压测量
在传统的溴化物水溶液(H 2 O + LiBr)中,提出了一种离子液体(IL)1-乙基-3-甲基咪唑碘[Emim] I和1,3-二甲基咪唑碘[Dmim] I。吸收式制冷循环。在350.78至414.19 K和H 2的温度下,两个三元系统H 2 O + LiBr + [Emim] I(质量比LiBr / [Emim] I = 4)的蒸气压数据通过沸点法报道了在350.69至409.63K的温度下的O + LiBr + [Dmim] I(质量比LiBr / [Dmim] I = 2)。吸收剂质量分数分别为0.30至0.57和0.30至0.60。使用Antoine型方程式对测量数据进行关联,测量数据与回归数据之间的平均绝对相对偏差(AARD%)为0.54和0.39%。与具有相同有机阳离子的IL混合物的蒸气压相比,蒸气压顺序为LiBr + [Emim] I + H 2 O <LiBr + [Emim] Cl + H 2 O <LiBr + [Emim] Ac + H 2 O和LiBr + [Dmim] I + H 2 O <LiBr + [Dmim] Cl + H 2 O <LiBr + [Dmim] BF 4 + H 2O.在实验条件下,碘阴离子(I的亲水性- )比那些氯阴离子(CL的更好- ),乙酸根阴离子(AC - ),和四氟硼酸阴离子(BF 4 - )。较低的蒸气压意味着更强的吸湿性能。所提出的三元混合物具有较低的蒸气压并且可以改善吸收系统的吸收效果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号