当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Phase Equilibrium of the Aqueous Ternary Systems (MgCl2 + MgB4O7 + H2O) and (MgCl2 + Mg2B6O11 + H2O) at 288.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jced.9b00852 Haiwen Ge 1, 2 , Hongjun Yang 1, 2 , Min Wang 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.jced.9b00852 Haiwen Ge 1, 2 , Hongjun Yang 1, 2 , Min Wang 1, 2
Affiliation
|
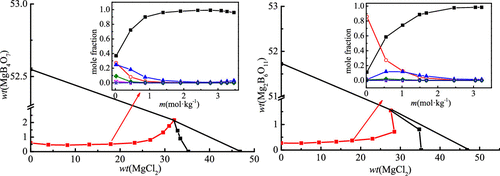
The solubility isotherms of the two ternary systems (MgCl2 + MgB4O7 + H2O) and (MgCl2 + Mg2B6O11 + H2O) were determined using the isothermal dissolution equilibrium method at 288.15 K. Solubilities, acidities, and densities of the liquid phase were determined in detail experimentally. According to the experimental data, the phase diagram and the relationship between density and composition were given. The results indicate that the ternary system (MgCl2 + MgB4O7 + H2O) is a simple saturated type, and double salt or solid solution are not formed at 288.15 K. There are one invariant point corresponding to (bischofite + magnesium borate), two isothermal dissolution curves, and two crystallization regions in the ternary system. There are one invariant point corresponding to bischofite and inderite, two isothermal dissolution curves, and two crystallization regions including bischofite and inderite in the ternary system (MgCl2 + Mg2B6O11 + H2O) at 288.15 K. It was found that the crystallization region area of magnesium borate was larger than that of bischofite, indicating that the solubility of bischofite was larger in the two systems. The distribution of polyborate species in magnesium borate isothermal dissolution curves varying with magnesium chloride molality was calculated by the Newton iteration method. The density data were calculated using the empirical formula, and the calculated results agree well with the experimental values.
中文翻译:

三元水体系(MgCl 2 + MgB 4 O 7 + H 2 O)和(MgCl 2 + Mg 2 B 6 O 11 + H 2 O)在288.15 K时的固液平衡
使用等温溶解平衡法在288.15 K下确定两个三元体系(MgCl 2 + MgB 4 O 7 + H 2 O)和(MgCl 2 + Mg 2 B 6 O 11 + H 2 O)的溶解度等温线。通过实验详细确定液相的酸度和密度。根据实验数据,给出了相图及密度与组成的关系。结果表明三元体系(MgCl 2 + MgB 4 O 7 + H 2O)是简单的饱和类型,在288.15 K时不形成复盐或固溶体。三元体系中有一个对应于(重钙铁矿+硼酸镁)的不变点,两个等温溶解曲线和两个结晶区。在三元体系中有一个不变点对应于重晶石和Inderite,两条等温溶解曲线,和两个结晶区包括Bischofite和Inderite(MgCl 2 + Mg 2 B 6 O 11 + H 2O)在288.15K。发现硼酸镁的结晶区域面积大于联镁矾的结晶区域,这表明联镁矾在两种体系中的溶解度较大。用牛顿迭代法计算了硼酸镁等温溶解曲线中多硼酸盐种类的分布,该曲线随氯化镁的摩尔浓度而变化。利用经验公式计算出密度数据,计算结果与实验值吻合良好。
更新日期:2020-01-07
中文翻译:

三元水体系(MgCl 2 + MgB 4 O 7 + H 2 O)和(MgCl 2 + Mg 2 B 6 O 11 + H 2 O)在288.15 K时的固液平衡
使用等温溶解平衡法在288.15 K下确定两个三元体系(MgCl 2 + MgB 4 O 7 + H 2 O)和(MgCl 2 + Mg 2 B 6 O 11 + H 2 O)的溶解度等温线。通过实验详细确定液相的酸度和密度。根据实验数据,给出了相图及密度与组成的关系。结果表明三元体系(MgCl 2 + MgB 4 O 7 + H 2O)是简单的饱和类型,在288.15 K时不形成复盐或固溶体。三元体系中有一个对应于(重钙铁矿+硼酸镁)的不变点,两个等温溶解曲线和两个结晶区。在三元体系中有一个不变点对应于重晶石和Inderite,两条等温溶解曲线,和两个结晶区包括Bischofite和Inderite(MgCl 2 + Mg 2 B 6 O 11 + H 2O)在288.15K。发现硼酸镁的结晶区域面积大于联镁矾的结晶区域,这表明联镁矾在两种体系中的溶解度较大。用牛顿迭代法计算了硼酸镁等温溶解曲线中多硼酸盐种类的分布,该曲线随氯化镁的摩尔浓度而变化。利用经验公式计算出密度数据,计算结果与实验值吻合良好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号