Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.comptc.2019.112700 Hamid Saeidian , Masomeh Ramezannejad , Salman Taheri , Zohreh Mirjafary
|
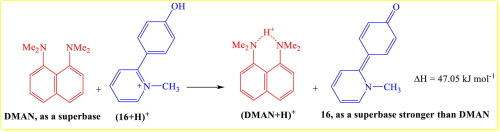
The gas phase proton affinity (PA) and basicity (GB) of a series of extended π-systems, possessing carbonyl as the most basic sites, were calculated using the DFT-B3LYP theoretical method. The backbone of the polycyclic π-electron networks contains either pyrone, pyrolidine or thiopyrane substructures, which can be in α or λ isomer forms. The PAs of designed molecules were reported in the range of 868-1089 kJ mol-1, indicating that some of the molecules have the basicity higher than 1,8-bis(dimethylamino) naphthalene. Such high basicity is a consequence of stabilization in protonated forms, due to the formation of the six-membered aromatic rings upon protonation which stabilize the positive charge. The aromaticity indices of the rings before and after protonation were calculated. The results show that aromaticity indices in the protonated form of the designed molecules is significantly higher than the neutral one.
中文翻译:

基于扩展π系统的鲁棒有机碱的定制:羰基碱度的密度泛函理论研究
利用DFT-B3LYP理论方法计算了一系列以羰基为最碱性位的扩展π系统的气相质子亲和力(PA)和碱度(GB)。多环π电子网络的主链包含吡喃,吡咯烷或噻喃基亚结构,其可以为α或λ异构体形式。据报道设计分子的PA范围为868-1089 kJ mol -1,表明某些分子的碱度高于1,8-双(二甲基氨基)萘。如此高的碱性是质子化形式稳定的结果,这是由于质子化时形成了六元芳环,使正电荷稳定。计算了质子化前后环的芳香指数。结果表明,所设计分子的质子化形式的芳香性指数显着高于中性分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号