Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the Vesicular Stomatitis Virus L Protein in Complex with Its Phosphoprotein Cofactor.
Cell Reports ( IF 7.5 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.celrep.2019.12.024 Simon Jenni 1 , Louis-Marie Bloyet 2 , Ruben Diaz-Avalos 3 , Bo Liang 4 , Sean P J Whelan 5 , Nikolaus Grigorieff 6 , Stephen C Harrison 7
Cell Reports ( IF 7.5 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.celrep.2019.12.024 Simon Jenni 1 , Louis-Marie Bloyet 2 , Ruben Diaz-Avalos 3 , Bo Liang 4 , Sean P J Whelan 5 , Nikolaus Grigorieff 6 , Stephen C Harrison 7
Affiliation
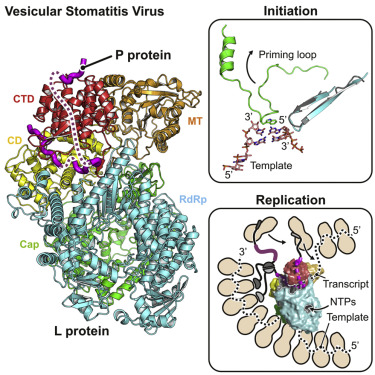
|
The large (L) proteins of non-segmented, negative-strand RNA viruses are multifunctional enzymes that produce capped, methylated, and polyadenylated mRNA and replicate the viral genome. A phosphoprotein (P), required for efficient RNA-dependent RNA polymerization from the viral ribonucleoprotein (RNP) template, regulates the function and conformation of the L protein. We report the structure of vesicular stomatitis virus L in complex with its P cofactor determined by electron cryomicroscopy at 3.0 Å resolution, enabling us to visualize bound segments of P. The contacts of three P segments with multiple L domains show how P induces a closed, compact, initiation-competent conformation. Binding of P to L positions its N-terminal domain adjacent to a putative RNA exit channel for efficient encapsidation of newly synthesized genomes with the nucleoprotein and orients its C-terminal domain to interact with an RNP template. The model shows that a conserved tryptophan in the priming loop can support the initiating 5' nucleotide.
中文翻译:

水疱性口炎病毒 L 蛋白及其磷蛋白辅因子复合物的结构。
非分段负链 RNA 病毒的大 (L) 蛋白是多功能酶,可产生加帽、甲基化和聚腺苷酸化 mRNA 并复制病毒基因组。磷蛋白 (P) 是病毒核糖核蛋白 (RNP) 模板有效依赖 RNA 聚合所需的磷蛋白 (P),它调节 L 蛋白的功能和构象。我们报告了水疱性口炎病毒 L 及其 P 辅因子复合物的结构,通过电子冷冻显微镜以 3.0 Å 分辨率测定,使我们能够可视化 P 的结合片段。三个 P 片段与多个 L 结构域的接触显示了 P 如何诱导闭合、紧凑的、具有引发能力的构象。P 与 L 的结合将其 N 端结构域定位在邻近假定的 RNA 出口通道的位置,以便将新合成的基因组与核蛋白有效包壳,并定向其 C 端结构域以与 RNP 模板相互作用。该模型表明,引发环中的保守色氨酸可以支持起始 5' 核苷酸。
更新日期:2020-01-07
中文翻译:

水疱性口炎病毒 L 蛋白及其磷蛋白辅因子复合物的结构。
非分段负链 RNA 病毒的大 (L) 蛋白是多功能酶,可产生加帽、甲基化和聚腺苷酸化 mRNA 并复制病毒基因组。磷蛋白 (P) 是病毒核糖核蛋白 (RNP) 模板有效依赖 RNA 聚合所需的磷蛋白 (P),它调节 L 蛋白的功能和构象。我们报告了水疱性口炎病毒 L 及其 P 辅因子复合物的结构,通过电子冷冻显微镜以 3.0 Å 分辨率测定,使我们能够可视化 P 的结合片段。三个 P 片段与多个 L 结构域的接触显示了 P 如何诱导闭合、紧凑的、具有引发能力的构象。P 与 L 的结合将其 N 端结构域定位在邻近假定的 RNA 出口通道的位置,以便将新合成的基因组与核蛋白有效包壳,并定向其 C 端结构域以与 RNP 模板相互作用。该模型表明,引发环中的保守色氨酸可以支持起始 5' 核苷酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号