Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TRIM32/USP11 Balances ARID1A Stability and the Oncogenic/Tumor-Suppressive Status of Squamous Cell Carcinoma.
Cell Reports ( IF 7.5 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.celrep.2019.12.017 Qingyu Luo 1 , Xiaowei Wu 1 , Yabing Nan 1 , Wan Chang 1 , Pengfei Zhao 1 , Yiping Zhang 1 , Dan Su 2 , Zhihua Liu 1
Cell Reports ( IF 7.5 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.celrep.2019.12.017 Qingyu Luo 1 , Xiaowei Wu 1 , Yabing Nan 1 , Wan Chang 1 , Pengfei Zhao 1 , Yiping Zhang 1 , Dan Su 2 , Zhihua Liu 1
Affiliation
|
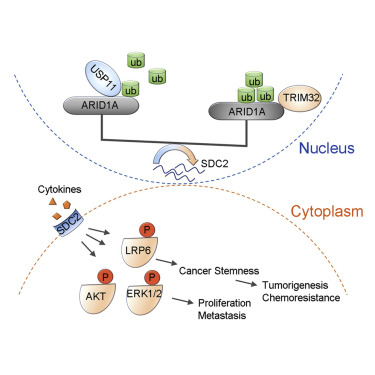
Squamous cell carcinoma (SCC) is an aggressive epithelial malignancy, yet the molecular mechanisms underlying SCC development are elusive. ARID1A is frequently mutated in various cancer types, but both mutation rates and expression levels of ARID1A are ubiquitously low in SCCs. Here, we reveal that excessive protein degradation mediated by the ubiquitin-proteasome system (UPS) contributes to the loss of ARID1A expression in SCC. We identify that the E3 ligase TRIM32 and the deubiquitinase USP11 play key roles in controlling ARID1A stability. TRIM32 depletion inhibits SCC cell proliferation, metastasis, and chemoresistance by stabilizing ARID1A, while USP11 depletion promotes SCC development by promoting ARID1A degradation. We show that syndecan-2 (SDC2) is the downstream target of both ARID1A and USP11 and that SDC2 depletion abolishes the oncogenic function of ARID1A loss. In summary, our data reveal UPS-mediated protein degradation as a mechanism underlying ARID1A loss and propose an important role for the TRIM32/USP11-ARID1A-SDC2 axis in SCC.
中文翻译:

TRIM32 / USP11平衡了ARID1A稳定性和鳞状细胞癌的致癌/肿瘤抑制状态。
鳞状细胞癌(SCC)是一种侵袭性上皮恶性肿瘤,但其潜在的分子机制尚不清楚。ARID1A在各种类型的癌症中经常发生突变,但在SCC中ARID1A的突变率和表达水平普遍较低。在这里,我们揭示了由遍在蛋白-蛋白酶体系统(UPS)介导的过度蛋白质降解导致SCC中ARID1A表达的丧失。我们确定E3连接酶TRIM32和去泛素酶USP11在控制ARID1A稳定性中起关键作用。TRIM32耗竭通过稳定ARID1A抑制SCC细胞增殖,转移和化学耐药性,而USP11耗竭通过促进ARID1A降解促进SCC发育。我们表明syndecan-2(SDC2)是ARID1A和USP11的下游目标,并且SDC2的消耗消除了ARID1A丢失的致癌功能。总而言之,我们的数据揭示了UPS介导的蛋白质降解是ARID1A缺失的潜在机制,并提出了SCC中TRIM32 / USP11-ARID1A-SDC2轴的重要作用。
更新日期:2020-01-07
中文翻译:

TRIM32 / USP11平衡了ARID1A稳定性和鳞状细胞癌的致癌/肿瘤抑制状态。
鳞状细胞癌(SCC)是一种侵袭性上皮恶性肿瘤,但其潜在的分子机制尚不清楚。ARID1A在各种类型的癌症中经常发生突变,但在SCC中ARID1A的突变率和表达水平普遍较低。在这里,我们揭示了由遍在蛋白-蛋白酶体系统(UPS)介导的过度蛋白质降解导致SCC中ARID1A表达的丧失。我们确定E3连接酶TRIM32和去泛素酶USP11在控制ARID1A稳定性中起关键作用。TRIM32耗竭通过稳定ARID1A抑制SCC细胞增殖,转移和化学耐药性,而USP11耗竭通过促进ARID1A降解促进SCC发育。我们表明syndecan-2(SDC2)是ARID1A和USP11的下游目标,并且SDC2的消耗消除了ARID1A丢失的致癌功能。总而言之,我们的数据揭示了UPS介导的蛋白质降解是ARID1A缺失的潜在机制,并提出了SCC中TRIM32 / USP11-ARID1A-SDC2轴的重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号