当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and structure-activity relationship studies of novel triazole agents with strong antifungal activity against Aspergillus fumigatus.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.bmcl.2020.126951 Zichao Ding 1 , Tingjunhong Ni 2 , Fei Xie 1 , Yumeng Hao 1 , Shichong Yu 1 , Xiaoyun Chai 1 , Yongsheng Jin 1 , Ting Wang 1 , Yuanying Jiang 2 , Dazhi Zhang 1
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.bmcl.2020.126951 Zichao Ding 1 , Tingjunhong Ni 2 , Fei Xie 1 , Yumeng Hao 1 , Shichong Yu 1 , Xiaoyun Chai 1 , Yongsheng Jin 1 , Ting Wang 1 , Yuanying Jiang 2 , Dazhi Zhang 1
Affiliation
|
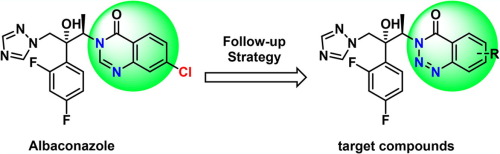
The incidence of invasive fungal infections has dramatically increased for several decades. In order to discover novel antifungal agents with broad spectrum and anti-Aspergillus efficacy, a series of novel triazole derivatives containing 1,2,3-benzotriazin-4-one was designed and synthesized. Most of the compounds exhibited stronger in vitro antifungal activities against tested fungi than fluconazole. Moreover, 6m showed comparable antifungal activity against seven pathogenic strains as voriconazole and albaconazole, especially against Aspergillus fumigatus (MIC = 0.25 μg/ml), and displayed moderate antifungal activity against fluconazole-resistant strains of Candida albicans. A clear SAR study indicated that compounds with groups at the 7-position resulted in novel antifungal triazoles with more effectiveness and a broader-spectrum.
中文翻译:

对烟曲霉具有较强抗真菌活性的新型三唑试剂的设计,合成和构效关系研究。
几十年来,侵袭性真菌感染的发生率急剧上升。为了发现具有广谱和抗曲霉功效的新型抗真菌剂,设计并合成了一系列含有1,2,3-苯并三嗪-4-酮的新型三唑衍生物。与氟康唑相比,大多数化合物对被测真菌表现出更强的体外抗真菌活性。此外,6m对伏立康唑和阿尔巴康唑的7种致病菌株,尤其是对烟曲霉(MIC = 0.25μg/ ml)表现出可比的抗真菌活性,对耐氟康唑的白色念珠菌菌株具有中等的抗真菌活性。一项清晰的SAR研究表明,在7位上带有基团的化合物可产生新型抗真菌三唑,具有更高的效力和更广的光谱范围。
更新日期:2020-01-07
中文翻译:

对烟曲霉具有较强抗真菌活性的新型三唑试剂的设计,合成和构效关系研究。
几十年来,侵袭性真菌感染的发生率急剧上升。为了发现具有广谱和抗曲霉功效的新型抗真菌剂,设计并合成了一系列含有1,2,3-苯并三嗪-4-酮的新型三唑衍生物。与氟康唑相比,大多数化合物对被测真菌表现出更强的体外抗真菌活性。此外,6m对伏立康唑和阿尔巴康唑的7种致病菌株,尤其是对烟曲霉(MIC = 0.25μg/ ml)表现出可比的抗真菌活性,对耐氟康唑的白色念珠菌菌株具有中等的抗真菌活性。一项清晰的SAR研究表明,在7位上带有基团的化合物可产生新型抗真菌三唑,具有更高的效力和更广的光谱范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号