当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A short divergent approach to highly substituted carbazoles and β-carbolines via in situ-generated diketoindoles
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.tetlet.2020.151597 Martin Untergehrer , Franz Bracher
中文翻译:

通过原位生成的二酮吲哚快速取代高度取代的咔唑和β-咔啉的方法
更新日期:2020-01-07
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.tetlet.2020.151597 Martin Untergehrer , Franz Bracher
|
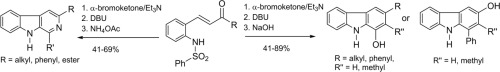
Based on an aza-alkylation/Michael addition cascade reaction developed by Kim and co-workers we have developed divergent cascade reactions leading to either highly substituted 1-hydroxycarbazoles, 3-hydroxycarbazoles or β-carbolines, starting from readily accessible ortho-arylsulfonylaminobenzaldehydes. Olefination of the aldehyde functionality by aldol condensation or Wittig olefination gave reactive enone intermediates, which underwent the cascade reactions, either in two steps or in one-pot conversions, to give hydroxycarbazoles or complex β-carbolines.
中文翻译:

通过原位生成的二酮吲哚快速取代高度取代的咔唑和β-咔啉的方法
基于Kim和他的同事开发的氮杂烷基化/迈克尔加成级联反应,我们开发了不同的级联反应,从容易获得的邻芳基磺酰基氨基苯甲醛开始,导致了高度取代的1-羟基咔唑,3-羟基咔唑或β-咔啉。通过醛醇缩合或维蒂希(Wittig)烯化反应对醛官能度进行烯烃化,得到反应性烯酮中间体,该中间体以两步或一锅转化进行级联反应,得到羟基咔唑或复杂的β-咔啉。



























 京公网安备 11010802027423号
京公网安备 11010802027423号