Energy Storage Materials ( IF 20.4 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.ensm.2020.01.002 Huanxin Li , Shuai Ma , Jiawen Li , Fuyu Liu , Haihui Zhou , Zhongyuan Huang , Shuqiang Jiao , Yafei Kuang
|
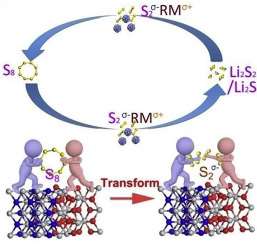
Lithium-sulfur (Li-S) battery is one of the most promising energy storage devices. However, the development of Li-S battery is seriously hindered by the “shuttle effect” of polysulfides. Up to now, almost in all the researches related to sulfur cathode, the polysulfide motion restricting strategy is used to suppress the “shuttle effect”. However, this issue still cannot be thoroughly solved. Here, we report a new polysulfide generation restricting strategy to eliminate the “shuttle effect” in Li-S batteries by turning the S8 molecules into stably adsorbed small sulfur species with suitable solid redox mediators (RMs) to generate an S2σ--RMσ+ at the very beginning. In this way, the mediators (S2σ--RMσ+) are reduced into Li2S2 and Li2S directly without soluble polysulfide forming during the discharging process. Therefore, the “shuttle effect” of polysulfides (Li2S8, Li2S6, and Li2S4) is absolutely eliminated. This new polysulfide generation restricting strategy is realized by using a TiOxNy-TiO2 quantum [email protected] composite ([email protected]) as a sulfur host. The [email protected] is proved to be an efficient RM to convert S8 molecules into stably adsorbed S2σ--RMσ+ species, eliminating the formation of lithium polysulfide completely. Owing to the new mechanism, the Li-S battery with [email protected] host achieves a capacity of 869 mA h g-1 (96% of the initial capacity) after 200 cycles with a low capacity decay of 0.02% per cycle. This strategy provides a new way to thoroughly solve the “shuttle effect” in Li-S batteries.
中文翻译:

改变反应机理消除锂硫电池的穿梭效应
锂硫(Li-S)电池是最有前途的储能设备之一。但是,多硫化物的“穿梭效应”严重阻碍了锂硫电池的发展。迄今为止,几乎所有与硫阴极有关的研究都采用多硫化物运动限制策略来抑制“穿梭效应”。但是,此问题仍然无法彻底解决。这里,我们报告一个新的多硫化物生成限制策略通过转动在S,以消除在栗-S电池“穿梭效应” 8与合适的固体氧化还原介体(RMS)分子进入稳定地吸附小硫物质,以产生一个S 2 σ- - RM σ+在开始的时候。以这种方式,所述介体(S 2 σ- -RMσ+)在放电过程中直接还原成Li 2 S 2和Li 2 S而不会形成可溶性多硫化物。因此,绝对消除了多硫化物(Li 2 S 8,Li 2 S 6和Li 2 S 4)的“穿梭效应” 。这种新的多硫化物生成限制策略是通过使用TiO x N y -TiO 2量子[受电子邮件保护的]复合材料([受电子邮件保护的])作为硫主体实现的。事实证明,[电子邮件保护]是将S 8分子转化为稳定吸附的S 2的有效RM。σ- -RM σ+物种,消除多硫化锂的形成完全。由于采用了新的机制,带有[电子邮件保护]主机的Li-S电池在200个循环后达到869 mA hg -1的容量(初始容量的96%),每个循环的容量衰减为0.02%。该策略提供了一种新方法,可以彻底解决Li-S电池的“穿梭效应”。



























 京公网安备 11010802027423号
京公网安备 11010802027423号