当前位置:
X-MOL 学术
›
Spectrochim. Acta. A Mol. Biomol. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidation of binding mechanism of dibutyl phthalate on bovine serum albumin by spectroscopic analysis and molecular docking method.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.saa.2020.118044 Lei Wang 1 , Jianfang Dong 2 , Rui Li 1 , Peiran Zhao 1 , Jinming Kong 3 , Lianzhi Li 1
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.saa.2020.118044 Lei Wang 1 , Jianfang Dong 2 , Rui Li 1 , Peiran Zhao 1 , Jinming Kong 3 , Lianzhi Li 1
Affiliation
|
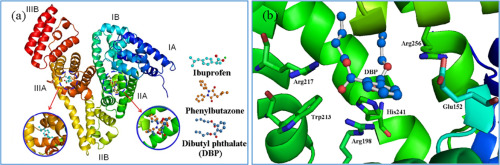
Dibutyl phthalate has been illegally used in beverages and directly affects the human health. Herein, the interaction occurred between dibutyl phthalate and bovine serum albumin was studied. The experimental results demonstrated that dibutyl phthalate could bind to bovine serum albumin and statically quench the intrinsic fluorescence of this protein. Circular dichroism measurements proved that the binding of dibutyl phthalate would lead to an obvious decrease of α-helix content in the bovine serum albumin. Molecular docking analysis clarified the fluorescence quenching mechanism, size distribution and zeta potential variation, conformational change of BSA, the site marker competitive fluorescence quenching and the interaction mechanism of dibutyl phthalate to bovine serum albumin. This work provided a useful information for the binding of dibutyl phthalate to protein.
中文翻译:

通过光谱分析和分子对接方法阐明邻苯二甲酸二丁酯与牛血清白蛋白的结合机理。
邻苯二甲酸二丁酯已被非法用于饮料中,直接影响人体健康。在此,研究了邻苯二甲酸二丁酯与牛血清白蛋白之间的相互作用。实验结果表明邻苯二甲酸二丁酯可以与牛血清白蛋白结合并静态淬灭该蛋白的固有荧光。圆二色性测量证明,邻苯二甲酸二丁酯的结合将导致牛血清白蛋白中α-螺旋含量的明显降低。分子对接分析阐明了BSA的荧光猝灭机理,大小分布和ζ电位变化,构象变化,位点标记竞争性荧光猝灭以及邻苯二甲酸二丁酯与牛血清白蛋白的相互作用机理。
更新日期:2020-01-07
中文翻译:

通过光谱分析和分子对接方法阐明邻苯二甲酸二丁酯与牛血清白蛋白的结合机理。
邻苯二甲酸二丁酯已被非法用于饮料中,直接影响人体健康。在此,研究了邻苯二甲酸二丁酯与牛血清白蛋白之间的相互作用。实验结果表明邻苯二甲酸二丁酯可以与牛血清白蛋白结合并静态淬灭该蛋白的固有荧光。圆二色性测量证明,邻苯二甲酸二丁酯的结合将导致牛血清白蛋白中α-螺旋含量的明显降低。分子对接分析阐明了BSA的荧光猝灭机理,大小分布和ζ电位变化,构象变化,位点标记竞争性荧光猝灭以及邻苯二甲酸二丁酯与牛血清白蛋白的相互作用机理。










































 京公网安备 11010802027423号
京公网安备 11010802027423号