当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the molecular origins of fibrin's elastomeric properties by in situ X-ray scattering.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.actbio.2020.01.002 Bart E Vos 1 , Cristina Martinez-Torres 2 , Federica Burla 3 , John W Weisel 4 , Gijsje H Koenderink 2
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.actbio.2020.01.002 Bart E Vos 1 , Cristina Martinez-Torres 2 , Federica Burla 3 , John W Weisel 4 , Gijsje H Koenderink 2
Affiliation
|
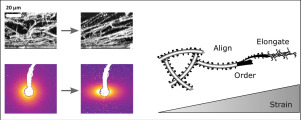
Fibrin is an elastomeric protein forming highly extensible fiber networks that provide the scaffold of blood clots. Here we reveal the molecular mechanisms that explain the large extensibility of fibrin networks by performing in situ small angle X-ray scattering measurements while applying a shear deformation. We simultaneously measure shear-induced alignment of the fibers and changes in their axially ordered molecular packing structure. We show that fibrin networks exhibit distinct structural responses that set in consecutively as the shear strain is increased. They exhibit an entropic response at small strains (<5%), followed by progressive fiber alignment (>25% strain) and finally changes in the fiber packing structure at high strain (>100%). Stretching reduces the fiber packing order and slightly increases the axial periodicity, indicative of molecular unfolding. However, the axial periodicity changes only by 0.7%, much less than the 80% length increase of the fibers, suggesting that fiber elongation mainly stems from uncoiling of the natively disordered αC-peptide linkers that laterally bond the molecules. Upon removal of the load, the network structure returns to the original isotropic state, but the fiber structure becomes more ordered and adopts a smaller packing periodicity compared to the original state. We conclude that the hierarchical packing structure of fibrin fibers, with built-in disorder, makes the fibers extensible and allows for mechanical annealing. Our results provide a basis for interpreting the molecular basis of haemostatic and thrombotic disorders associated with clotting and provide inspiration to design resilient bio-mimicking materials. STATEMENT OF SIGNIFICANCE: Fibrin provides structural integrity to blood clots and is also widely used as a scaffold for tissue engineering. To fulfill their biological functions, fibrin networks have to be simultaneously compliant like skin and resilient against rupture. Here, we unravel the structural origin underlying this remarkable mechanical behaviour. To this end, we performed in situ measurements of fibrin structure across multiple length scales by combining X-ray scattering with shear rheology. Our findings show that fibrin sustains large strains by undergoing a sequence of structural changes on different scales with increasing strain levels. This demonstrates new mechanistic aspects of an important biomaterial's structure and its mechanical function, and serves as an example in the design of biomimicking materials.
中文翻译:

通过原位X射线散射揭示纤维蛋白弹性特性的分子起源。
纤维蛋白是形成高度可扩展的纤维网络的弹性蛋白,该纤维网络提供了血块的支架。在这里,我们通过施加剪切变形的同时进行原位小角度X射线散射测量,揭示了解释纤维蛋白网络可扩展性的分子机制。我们同时测量了剪切引起的纤维排列和其轴向有序分子堆积结构的变化。我们显示血纤蛋白网络表现出不同的结构响应,随着剪切应变的增加而连续设置。它们在小应变(<5%)时表现出熵响应,然后逐渐进行纤维排列(> 25%应变),最后在高应变(> 100%)时纤维堆积结构发生变化。拉伸会降低纤维堆积顺序,并略微增加轴向周期性,指示分子展开。但是,轴向周期性仅变化0.7%,远小于纤维长度增加80%的程度,这表明纤维伸长主要来自与分子横向键合的天然无序αC肽接头的开卷。除去负载后,网络结构返回到原始的各向同性状态,但是与原始状态相比,纤维结构变得更有序,并且采用更小的填充周期。我们得出的结论是,纤维蛋白纤维的分层堆积结构具有固有的无序性,使纤维可扩展并允许机械退火。我们的结果为解释与凝血相关的止血和血栓形成疾病的分子基础提供了基础,并为设计弹性的仿生材料提供了灵感。意义声明:纤维蛋白可为血凝块提供结构完整性,也被广泛用作组织工程的支架。为了履行其生物学功能,纤维蛋白网络必须像皮肤一样同时顺应性,并具有抗破裂的弹性。在这里,我们揭示了这种非凡的机械行为背后的结构起源。为此,我们通过结合X射线散射和剪切流变学进行了跨多个长度尺度的纤维蛋白结构的原位测量。我们的研究结果表明,纤维蛋白通过承受不同程度的结构变化(随着应变水平的增加)而承受大的应变。这证明了重要生物材料的结构及其机械功能的新的机械方面,并在仿生材料的设计中作为示例。纤维蛋白为血凝块提供结构完整性,也被广泛用作组织工程的支架。为了履行其生物学功能,纤维蛋白网络必须像皮肤一样同时顺应性,并具有抗破裂的弹性。在这里,我们揭示了这种非凡的机械行为背后的结构起源。为此,我们通过结合X射线散射和剪切流变学进行了跨多个长度尺度的纤维蛋白结构的原位测量。我们的研究结果表明,纤维蛋白通过承受不同程度的结构变化(随着应变水平的增加)而承受大的应变。这证明了重要生物材料的结构及其机械功能的新的机械方面,并在仿生材料的设计中作为示例。纤维蛋白为血凝块提供结构完整性,也被广泛用作组织工程的支架。为了履行其生物学功能,纤维蛋白网络必须像皮肤一样同时顺应性,并具有抗破裂的弹性。在这里,我们揭示了这种非凡的机械行为背后的结构起源。为此,我们通过结合X射线散射和剪切流变学进行了跨多个长度尺度的纤维蛋白结构的原位测量。我们的研究结果表明,纤维蛋白通过承受不同程度的结构变化(随着应变水平的增加)而承受大的应变。这证明了重要生物材料的结构及其机械功能的新的机械方面,并在仿生材料的设计中作为示例。
更新日期:2020-01-07
中文翻译:

通过原位X射线散射揭示纤维蛋白弹性特性的分子起源。
纤维蛋白是形成高度可扩展的纤维网络的弹性蛋白,该纤维网络提供了血块的支架。在这里,我们通过施加剪切变形的同时进行原位小角度X射线散射测量,揭示了解释纤维蛋白网络可扩展性的分子机制。我们同时测量了剪切引起的纤维排列和其轴向有序分子堆积结构的变化。我们显示血纤蛋白网络表现出不同的结构响应,随着剪切应变的增加而连续设置。它们在小应变(<5%)时表现出熵响应,然后逐渐进行纤维排列(> 25%应变),最后在高应变(> 100%)时纤维堆积结构发生变化。拉伸会降低纤维堆积顺序,并略微增加轴向周期性,指示分子展开。但是,轴向周期性仅变化0.7%,远小于纤维长度增加80%的程度,这表明纤维伸长主要来自与分子横向键合的天然无序αC肽接头的开卷。除去负载后,网络结构返回到原始的各向同性状态,但是与原始状态相比,纤维结构变得更有序,并且采用更小的填充周期。我们得出的结论是,纤维蛋白纤维的分层堆积结构具有固有的无序性,使纤维可扩展并允许机械退火。我们的结果为解释与凝血相关的止血和血栓形成疾病的分子基础提供了基础,并为设计弹性的仿生材料提供了灵感。意义声明:纤维蛋白可为血凝块提供结构完整性,也被广泛用作组织工程的支架。为了履行其生物学功能,纤维蛋白网络必须像皮肤一样同时顺应性,并具有抗破裂的弹性。在这里,我们揭示了这种非凡的机械行为背后的结构起源。为此,我们通过结合X射线散射和剪切流变学进行了跨多个长度尺度的纤维蛋白结构的原位测量。我们的研究结果表明,纤维蛋白通过承受不同程度的结构变化(随着应变水平的增加)而承受大的应变。这证明了重要生物材料的结构及其机械功能的新的机械方面,并在仿生材料的设计中作为示例。纤维蛋白为血凝块提供结构完整性,也被广泛用作组织工程的支架。为了履行其生物学功能,纤维蛋白网络必须像皮肤一样同时顺应性,并具有抗破裂的弹性。在这里,我们揭示了这种非凡的机械行为背后的结构起源。为此,我们通过结合X射线散射和剪切流变学进行了跨多个长度尺度的纤维蛋白结构的原位测量。我们的研究结果表明,纤维蛋白通过承受不同程度的结构变化(随着应变水平的增加)而承受大的应变。这证明了重要生物材料的结构及其机械功能的新的机械方面,并在仿生材料的设计中作为示例。纤维蛋白为血凝块提供结构完整性,也被广泛用作组织工程的支架。为了履行其生物学功能,纤维蛋白网络必须像皮肤一样同时顺应性,并具有抗破裂的弹性。在这里,我们揭示了这种非凡的机械行为背后的结构起源。为此,我们通过结合X射线散射和剪切流变学进行了跨多个长度尺度的纤维蛋白结构的原位测量。我们的研究结果表明,纤维蛋白通过承受不同程度的结构变化(随着应变水平的增加)而承受大的应变。这证明了重要生物材料的结构及其机械功能的新的机械方面,并在仿生材料的设计中作为示例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号