当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox-responsive polyprodrug nanoparticles for targeted siRNA delivery and synergistic liver cancer therapy.
Biomaterials ( IF 12.8 ) Pub Date : 2020-01-06 , DOI: 10.1016/j.biomaterials.2020.119760 Senlin Li 1 , Phei Er Saw 2 , Chunhao Lin 2 , Yan Nie 2 , Wei Tao 3 , Omid C Farokhzad 3 , Lei Zhang 1 , Xiaoding Xu 2
Biomaterials ( IF 12.8 ) Pub Date : 2020-01-06 , DOI: 10.1016/j.biomaterials.2020.119760 Senlin Li 1 , Phei Er Saw 2 , Chunhao Lin 2 , Yan Nie 2 , Wei Tao 3 , Omid C Farokhzad 3 , Lei Zhang 1 , Xiaoding Xu 2
Affiliation
|
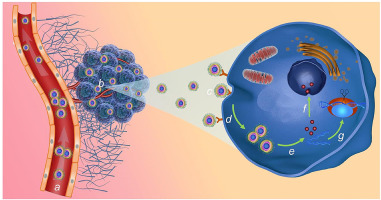
Combination therapy has been developed as an innovative modality for effective cancer therapy. However, the administration of combinatorial therapeutics is limited by the varying pharmacokinetics of different drugs. Although numerous nanoparticles (NPs) can synchronize the delivery of combinatorial therapeutics to tumor cells, their clinical translation is still challenged, which is partly due to the complexity to precisely control the loading of combinatorial therapeutics to maximize therapeutic efficacy and suboptimal NP properties. Herein, a new redox-responsive polyprodrug nanoplatform was developed for targeted siRNA delivery and synergistic cancer therapy. This NP platform is made with redox-responsive 10-hydroxycamptothecin (HCPT)-based polyprodrug (polyHCPT) as the inner core, amphiphilic lipid-poly (ethylene glycol) (lipid-PEG) as the outer shell, and lactobionic acid (LA) decoration on the surface. After siRNA loading and subsequent systemic administration, the resulting NP platform could accumulate in tumor tissues and target hepatoma cells via specific recognition between LA and asialoglycoprotein (ASGP) receptors. With the high concentration of glutathione (GSH) in the cytoplasm to break the disulfide bonds in the polyHCPT, intact HCPT molecules and encapsulated B-cell lymphoma 2 (Bcl-2) siRNA (siBcl-2) could be rapidly released, leading to the synergistic inhibition of tumor growth via the induction of apoptosis by HCPT and the concurrent silencing of the anti-apoptotic gene by siBcl-2.
中文翻译:

用于靶向 siRNA 递送和协同肝癌治疗的氧化还原反应性聚前体药物纳米颗粒。
联合疗法已发展成为有效癌症治疗的创新方式。然而,组合疗法的施用受到不同药物的不同药代动力学的限制。尽管许多纳米粒子 (NP) 可以同步将组合疗法递送至肿瘤细胞,但它们的临床转化仍然受到挑战,部分原因是精确控制组合疗法的负载以最大化治疗效果和次优 NP 特性的复杂性。在此,开发了一种新的氧化还原响应多聚前体药物纳米平台,用于靶向 siRNA 递送和协同癌症治疗。该NP平台以氧化还原反应性10-羟基喜树碱(HCPT)为基础的聚前体药物(polyHCPT)为内核,两亲性脂质聚乙二醇(lipid-PEG)为外壳,表面装饰有乳糖酸(LA)。在 siRNA 加载和随后的全身给药后,所得 NP 平台可以通过 LA 和去唾液酸糖蛋白 (ASGP) 受体之间的特异性识别在肿瘤组织中积累并靶向肝癌细胞。随着细胞质中高浓度的谷胱甘肽 (GSH) 破坏 polyHCPT 中的二硫键,完整的 HCPT 分子和封装的 B 细胞淋巴瘤 2 (Bcl-2) siRNA (siBcl-2) 可以快速释放,导致通过 HCPT 诱导细胞凋亡和 siBcl-2 同时沉默抗细胞凋亡基因协同抑制肿瘤生长。由此产生的 NP 平台可以通过 LA 和去唾液酸糖蛋白 (ASGP) 受体之间的特异性识别在肿瘤组织中积累并靶向肝癌细胞。随着细胞质中高浓度的谷胱甘肽 (GSH) 破坏 polyHCPT 中的二硫键,完整的 HCPT 分子和封装的 B 细胞淋巴瘤 2 (Bcl-2) siRNA (siBcl-2) 可以快速释放,导致通过 HCPT 诱导细胞凋亡和 siBcl-2 同时沉默抗细胞凋亡基因协同抑制肿瘤生长。由此产生的 NP 平台可以通过 LA 和去唾液酸糖蛋白 (ASGP) 受体之间的特异性识别在肿瘤组织中积累并靶向肝癌细胞。随着细胞质中高浓度的谷胱甘肽 (GSH) 破坏 polyHCPT 中的二硫键,完整的 HCPT 分子和封装的 B 细胞淋巴瘤 2 (Bcl-2) siRNA (siBcl-2) 可以快速释放,导致通过 HCPT 诱导细胞凋亡和 siBcl-2 同时沉默抗细胞凋亡基因协同抑制肿瘤生长。
更新日期:2020-01-06
中文翻译:

用于靶向 siRNA 递送和协同肝癌治疗的氧化还原反应性聚前体药物纳米颗粒。
联合疗法已发展成为有效癌症治疗的创新方式。然而,组合疗法的施用受到不同药物的不同药代动力学的限制。尽管许多纳米粒子 (NP) 可以同步将组合疗法递送至肿瘤细胞,但它们的临床转化仍然受到挑战,部分原因是精确控制组合疗法的负载以最大化治疗效果和次优 NP 特性的复杂性。在此,开发了一种新的氧化还原响应多聚前体药物纳米平台,用于靶向 siRNA 递送和协同癌症治疗。该NP平台以氧化还原反应性10-羟基喜树碱(HCPT)为基础的聚前体药物(polyHCPT)为内核,两亲性脂质聚乙二醇(lipid-PEG)为外壳,表面装饰有乳糖酸(LA)。在 siRNA 加载和随后的全身给药后,所得 NP 平台可以通过 LA 和去唾液酸糖蛋白 (ASGP) 受体之间的特异性识别在肿瘤组织中积累并靶向肝癌细胞。随着细胞质中高浓度的谷胱甘肽 (GSH) 破坏 polyHCPT 中的二硫键,完整的 HCPT 分子和封装的 B 细胞淋巴瘤 2 (Bcl-2) siRNA (siBcl-2) 可以快速释放,导致通过 HCPT 诱导细胞凋亡和 siBcl-2 同时沉默抗细胞凋亡基因协同抑制肿瘤生长。由此产生的 NP 平台可以通过 LA 和去唾液酸糖蛋白 (ASGP) 受体之间的特异性识别在肿瘤组织中积累并靶向肝癌细胞。随着细胞质中高浓度的谷胱甘肽 (GSH) 破坏 polyHCPT 中的二硫键,完整的 HCPT 分子和封装的 B 细胞淋巴瘤 2 (Bcl-2) siRNA (siBcl-2) 可以快速释放,导致通过 HCPT 诱导细胞凋亡和 siBcl-2 同时沉默抗细胞凋亡基因协同抑制肿瘤生长。由此产生的 NP 平台可以通过 LA 和去唾液酸糖蛋白 (ASGP) 受体之间的特异性识别在肿瘤组织中积累并靶向肝癌细胞。随着细胞质中高浓度的谷胱甘肽 (GSH) 破坏 polyHCPT 中的二硫键,完整的 HCPT 分子和封装的 B 细胞淋巴瘤 2 (Bcl-2) siRNA (siBcl-2) 可以快速释放,导致通过 HCPT 诱导细胞凋亡和 siBcl-2 同时沉默抗细胞凋亡基因协同抑制肿瘤生长。










































 京公网安备 11010802027423号
京公网安备 11010802027423号