当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurement and Modeling of the Solubility of N,N-Dibenzylhydroxylamine in 17 Solvents from T = 273.15 to 323.35 K and Thermodynamic Properties of Solution
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.jced.9b01028 Yajun Li 1 , Xiaofang Li 2 , Kui Wu 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.jced.9b01028 Yajun Li 1 , Xiaofang Li 2 , Kui Wu 3
Affiliation
|
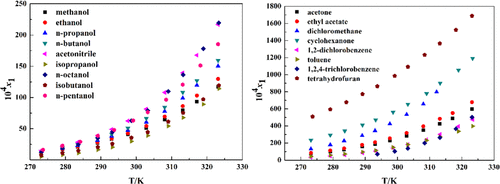
In this work, the solubility of N,N-dibenzylhydroxylamine (DBHA) in 17 pure solvents, including methanol, ethanol, n-propanol, n-butanol, acetone, ethyl acetate, dichloromethane, acetonitrile, isopropanol, n-octanol, cyclohexanone, 1,2-dichlorobenzene, toluene, isobutanol, n-pentanol, 1,2,4-trichlorobenzene, and tetrahydrofuran, was determined by a static gravimetric method with the temperature ranging from 273.15 to 323.35 K under atmospheric pressure. The solubility of DBHA was positively related to temperature in all selected solvents. Besides, the experimental solubility data were correlated with four thermodynamic models, i.e., the modified Apelblat equation, λh equation, nonrandom two-liquid (NRTL) equation, and the Wilson equation. The results indicated that all of the models could give satisfactory results and the NRTL equation provided the best fitting result. Furthermore, the dissolution thermodynamic parameters in the used solvents, including the Gibbs energy (ΔdisG), molar enthalpy (ΔdisH), and molar entropy (ΔdisS), were calculated according to the Wilson model, and it turned out that the dissolution process of DBHA was endothermic.
中文翻译:

N,N-二苄基羟胺在17种T = 273.15至323.35 K溶剂中的溶解度和溶液热力学性质的测量和建模
在这项工作中,N,N-二苄基羟胺(DBHA)在17种纯溶剂中的溶解度,包括甲醇,乙醇,正丙醇,正丁醇,丙酮,乙酸乙酯,二氯甲烷,乙腈,异丙醇,正辛醇,环己酮,通过静态重量法在大气压下以273.15至323.35 K的温度测定1,2-二氯苯,甲苯,异丁醇,正戊醇,1,2,4-三氯苯和四氢呋喃。在所有选定的溶剂中,DBHA的溶解度与温度呈正相关。此外,实验溶解度数据用4个热力学模型,即,修改后的方程Apelblat相关,λ ħ方程,非随机二液(NRTL)方程和Wilson方程。结果表明,所有模型都能给出满意的结果,NRTL方程提供了最佳拟合结果。此外,在所使用的溶剂中的溶解的热力学参数,包括Gibbs能(Δ DIS ģ),摩尔焓(Δ存款保险计划ħ),和摩尔熵(Δ存款保险计划小号),根据Wilson模型进行了计算,并且它变成了DBHA的溶解过程是吸热的。
更新日期:2020-01-06
中文翻译:

N,N-二苄基羟胺在17种T = 273.15至323.35 K溶剂中的溶解度和溶液热力学性质的测量和建模
在这项工作中,N,N-二苄基羟胺(DBHA)在17种纯溶剂中的溶解度,包括甲醇,乙醇,正丙醇,正丁醇,丙酮,乙酸乙酯,二氯甲烷,乙腈,异丙醇,正辛醇,环己酮,通过静态重量法在大气压下以273.15至323.35 K的温度测定1,2-二氯苯,甲苯,异丁醇,正戊醇,1,2,4-三氯苯和四氢呋喃。在所有选定的溶剂中,DBHA的溶解度与温度呈正相关。此外,实验溶解度数据用4个热力学模型,即,修改后的方程Apelblat相关,λ ħ方程,非随机二液(NRTL)方程和Wilson方程。结果表明,所有模型都能给出满意的结果,NRTL方程提供了最佳拟合结果。此外,在所使用的溶剂中的溶解的热力学参数,包括Gibbs能(Δ DIS ģ),摩尔焓(Δ存款保险计划ħ),和摩尔熵(Δ存款保险计划小号),根据Wilson模型进行了计算,并且它变成了DBHA的溶解过程是吸热的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号