当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Radical Species and Operating Parameters on the Degradation of Sulfapyridine Using a UV/Chlorine System
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.iecr.9b06228 Huaying Liu 1 , Biaojun Zhang 1 , Yingjie Li 1 , Qi Fang 1 , Zhichao Hou 1 , Senlin Tian 1 , Junjie Gu 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.iecr.9b06228 Huaying Liu 1 , Biaojun Zhang 1 , Yingjie Li 1 , Qi Fang 1 , Zhichao Hou 1 , Senlin Tian 1 , Junjie Gu 1
Affiliation
|
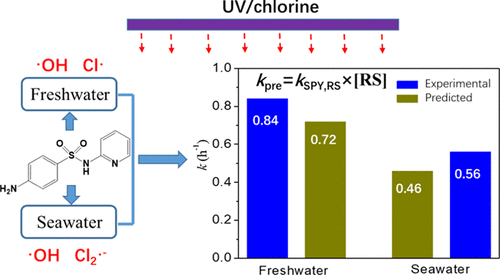
Degradation of sulfapyridine as a representative sulfonamide by the UV/chlorine process was investigated. Kinetic experiments revealed that aniline and sulfonamide moieties were sulfapyridine’s main reaction sites with •OH, and the aniline moiety was the preferable site for Cl• attack. Effects of four influence factors (pH; humic acid, HA; HCO3–; Cl–) on the degradation kinetics were explored by response surface methodology, and it was found that pH and HA have the highest level influence (81%) among all influence variables (linear, quadratic, and interactive terms), and low pH and concentrations of HA were beneficial for degradation. Approximately 70% of sulfapyridine degradation was observed within 60 min in the UV/chlorine system (pH = 5) without HA. A kinetic model considering steady-state concentrations of reactive radicals and their second-order rate constants is suitable for predicting sulfapyridine degradation, elucidating that •OH and Cl• are major radicals for sulfapyridine degradation in freshwater, and •OH and Cl2•– are responsible for the degradation in coastal seawater.
中文翻译:

自由基种类和操作参数对紫外/氯系统降解磺胺吡啶的影响
研究了通过紫外/氯气法降解磺胺吡啶作为代表性的磺酰胺。动力学实验表明,苯胺和磺酰胺部分是磺胺吡啶与• OH的主要反应位点,苯胺部分是Cl •进攻的优选位点。四个影响因素(pH;腐殖酸,HA; HCO 3 –; Cl –)通过响应面方法探索了对降解动力学的影响,发现pH和HA在所有影响变量(线性,二次和交互作用项)中具有最高水平的影响(81%),并且pH和HA浓度低对降解有益。在无HA的UV /氯系统(pH = 5)中,在60分钟内观察到约70%的磺胺吡啶降解。考虑反应基团的稳态浓度及其二级速率常数的动力学模型适用于预测磺胺吡啶的降解,阐明了• OH和Cl •是淡水中磺胺吡啶降解的主要自由基,以及• OH和Cl 2 •– 是造成沿海海水退化的原因。
更新日期:2020-01-17
中文翻译:

自由基种类和操作参数对紫外/氯系统降解磺胺吡啶的影响
研究了通过紫外/氯气法降解磺胺吡啶作为代表性的磺酰胺。动力学实验表明,苯胺和磺酰胺部分是磺胺吡啶与• OH的主要反应位点,苯胺部分是Cl •进攻的优选位点。四个影响因素(pH;腐殖酸,HA; HCO 3 –; Cl –)通过响应面方法探索了对降解动力学的影响,发现pH和HA在所有影响变量(线性,二次和交互作用项)中具有最高水平的影响(81%),并且pH和HA浓度低对降解有益。在无HA的UV /氯系统(pH = 5)中,在60分钟内观察到约70%的磺胺吡啶降解。考虑反应基团的稳态浓度及其二级速率常数的动力学模型适用于预测磺胺吡啶的降解,阐明了• OH和Cl •是淡水中磺胺吡啶降解的主要自由基,以及• OH和Cl 2 •– 是造成沿海海水退化的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号