当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
6-Nitrochrysene-Derived C8-2'-Deoxyadenosine Adduct: Synthesis of Site-Specific Oligodeoxynucleotides and Mutagenicity in Escherichia coli.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.chemrestox.9b00429 Brent V Powell 1 , Ashis K Basu 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.chemrestox.9b00429 Brent V Powell 1 , Ashis K Basu 1
Affiliation
|
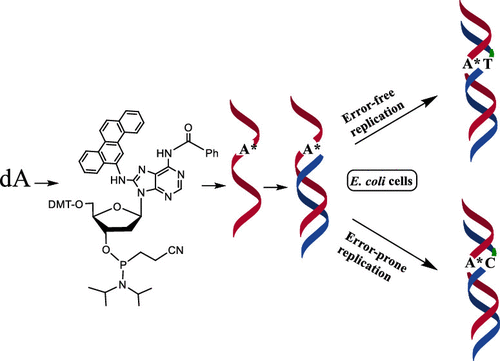
6-Nitrochrysene (6-NC), the most potent carcinogen evaluated by the newborn mouse assay, is metabolically activated by nitroreduction and a combination of ring oxidation and nitroreduction pathways. The nitroreduction pathway yields three major DNA adducts: at the C8 and N2 positions of 2'-deoxyguanosine (dG), N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC, and at the C8 position of 2'-deoxyadenosine (dA), N-(dA-8-yl)-6-AC. A nucleotide excision repair assay demonstrated that N-(dA-8-yl)-6-AC is repaired much more slowly than many other bulky DNA adducts, including the other DNA adducts formed by 6-NC. But neither the total synthesis nor evaluation of other biological activities of this dA adduct has ever been reported. Herein, we report a convenient synthesis of the 6-NC-derived dA adduct by employing the Buchwald-Hartwig coupling strategy, which provided a high yield of the protected N-(dA-8-yl)-6-AC. The deprotected nucleoside showed syn conformational preference by NMR spectroscopy. Following DMT protection of the 5'-hydroxyl, N-(dA-8-yl)-6-AC was converted to its 3'-phosphoramidite, which was used to prepare oligonucleotides containing a single N-(dA-8-yl)-6-AC adduct. Circular dichroism spectra of the adducted duplex showed only a slight departure from the B-DNA helix profile of the control duplex. The 15-mer N-(dA-8-yl)-6-AC oligonucleotide was used to construct a single-stranded plasmid vector containing a single adduct, which was replicated in Escherichia coli. Viability of the adducted construct was ∼60% of the control, indicating slower translesion synthesis of the adduct, which increased to nearly 90% upon induction of the SOS functions. Without SOS, the mutation frequency (MF) of the adduct was 5.2%, including 2.9% targeted and 2.3% semi-targeted mutations. With SOS, the targeted MF increased 3-fold to 9.0%, whereas semi-targeted mutation increased only marginally to 3.2%. The major type of targeted mutation was A*→G in both uninduced and SOS-induced cells.
中文翻译:

6-亚硝基衍生的C8-2'-脱氧腺苷加合物:特定位点的寡脱氧核苷酸的合成和致突变性
新生小鼠测定法评估的最强致癌物6-硝基丙烯(6-NC)通过硝基还原以及环氧化和硝基还原途径的组合被代谢激活。硝基还原途径产生三种主要的DNA加合物:在2'-脱氧鸟苷(dG),N-(dG-8-基)-6-AC和5-(dG-N2-基)-6-AC的C8和N2位置AC,并且在2'-脱氧腺苷(dA)的C8位置是N-(dA-8-yl)-6-AC。核苷酸切除修复测定表明,N-(dA-8-基)-6-AC的修复速度比许多其他大体积DNA加合物(包括由6-NC形成的其他DNA加合物)的修复速度要慢得多。但是,尚未报道过该dA加合物的总合成或其他生物学活性的评估。在此,我们报告了采用Buchwald-Hartwig偶联策略方便地合成6-NC衍生的dA加合物的方法,其提供了高产率的被保护的N-(dA-8-基)-6-AC。脱保护的核苷通过NMR光谱显示出顺式构象偏好。在5'-羟基的DMT保护之后,N-(dA-8-基)-6-AC转化为其3'-亚磷酰胺基,用于制备含有单个N-(dA-8-基)的寡核苷酸。 -6-AC加合物。加成双链体的圆二色性光谱显示仅与对照双链体的B-DNA螺旋线略有偏离。使用15-mer N-(dA-8-基)-6-AC寡核苷酸构建包含单加合物的单链质粒载体,该载体在大肠杆菌中复制。加合物构建体的存活力是对照的〜60%,表明加合物的跨病变合成较慢,在诱导SOS功能时其增加至近90%。没有SOS,加合物的突变频率(MF)为5.2%,包括2.9%的目标突变和2.3%的半目标突变。使用SOS,目标MF增加了3倍,达到9.0%,而半目标突变仅略有增加,达到了3.2%。在未诱导和SOS诱导的细胞中,靶向突变的主要类型是A *→G。
更新日期:2020-01-17
中文翻译:

6-亚硝基衍生的C8-2'-脱氧腺苷加合物:特定位点的寡脱氧核苷酸的合成和致突变性
新生小鼠测定法评估的最强致癌物6-硝基丙烯(6-NC)通过硝基还原以及环氧化和硝基还原途径的组合被代谢激活。硝基还原途径产生三种主要的DNA加合物:在2'-脱氧鸟苷(dG),N-(dG-8-基)-6-AC和5-(dG-N2-基)-6-AC的C8和N2位置AC,并且在2'-脱氧腺苷(dA)的C8位置是N-(dA-8-yl)-6-AC。核苷酸切除修复测定表明,N-(dA-8-基)-6-AC的修复速度比许多其他大体积DNA加合物(包括由6-NC形成的其他DNA加合物)的修复速度要慢得多。但是,尚未报道过该dA加合物的总合成或其他生物学活性的评估。在此,我们报告了采用Buchwald-Hartwig偶联策略方便地合成6-NC衍生的dA加合物的方法,其提供了高产率的被保护的N-(dA-8-基)-6-AC。脱保护的核苷通过NMR光谱显示出顺式构象偏好。在5'-羟基的DMT保护之后,N-(dA-8-基)-6-AC转化为其3'-亚磷酰胺基,用于制备含有单个N-(dA-8-基)的寡核苷酸。 -6-AC加合物。加成双链体的圆二色性光谱显示仅与对照双链体的B-DNA螺旋线略有偏离。使用15-mer N-(dA-8-基)-6-AC寡核苷酸构建包含单加合物的单链质粒载体,该载体在大肠杆菌中复制。加合物构建体的存活力是对照的〜60%,表明加合物的跨病变合成较慢,在诱导SOS功能时其增加至近90%。没有SOS,加合物的突变频率(MF)为5.2%,包括2.9%的目标突变和2.3%的半目标突变。使用SOS,目标MF增加了3倍,达到9.0%,而半目标突变仅略有增加,达到了3.2%。在未诱导和SOS诱导的细胞中,靶向突变的主要类型是A *→G。









































 京公网安备 11010802027423号
京公网安备 11010802027423号