当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of ZnO Nanostructures with Proteins: In Vitro Fibrillation/Antifibrillation Studies and in Silico Molecular Docking Simulations.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-01-13 , DOI: 10.1021/acschemneuro.9b00642 Kleoniki Giannousi 1 , George Geromichalos 1 , Dionysia Kakolyri 1 , Stefanos Mourdikoudis 2, 3 , Catherine Dendrinou-Samara 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-01-13 , DOI: 10.1021/acschemneuro.9b00642 Kleoniki Giannousi 1 , George Geromichalos 1 , Dionysia Kakolyri 1 , Stefanos Mourdikoudis 2, 3 , Catherine Dendrinou-Samara 1
Affiliation
|
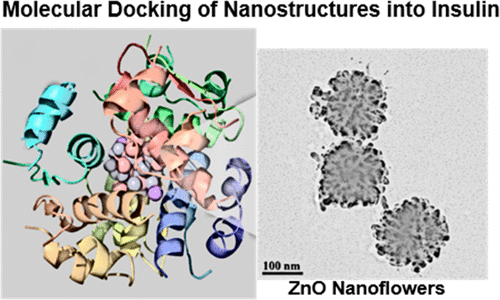
Protein amyloidosis is related to many neurological disorders. Nanoparticles (NPs) due to their small size can regulate both the polypeptide monomers/oligomers assembly into amyloid fibrils/plaques and the disintegration of the existent plaques. Herein, we have synthesized ZnO nanoflowers and polyol-coated ZnO NPs of relatively small size (40 nm) with cylindrical shape, through solvothermal and microwave-assisted routes, respectively. The effect of the different morphology of nanostructures on the fibrillation/antifibrillation process was monitored in bovine serum albumin (BSA) and human insulin (HI) by fluorescence Thioflavin T (ThT) measurements. Although both nanomaterials affected the amyloid formation mechanism as well as their disaggregation, ZnO nanoflowers with their sharp edges exhibited the greatest amyloid degradation rate in both model proteins (73% and 35%, respectively) and inhibited the most the insulin fibril growth, while restrained also the fibrillation process in the case of albumin solution. In silico molecular docking simulations on the crystal structure of BSA and HI were performed to analyze further the observed in vitro activity of ZnO nanostructures. The binding energy of ZnO NPs was found lower for BSA (-5.44), highlighting their ability to act as catalysts in the fibrillation process of albumin monomers.
中文翻译:

ZnO纳米结构与蛋白质的相互作用:体外原纤化/抗原纤化研究和计算机分子对接模拟。
蛋白质淀粉样变性病与许多神经系统疾病有关。纳米颗粒(NPs)由于其尺寸小,可以调节多肽单体/寡聚体组装成淀粉样原纤维/斑块以及现有斑块的崩解。在这里,我们分别通过溶剂热和微波辅助途径合成了圆柱形的ZnO纳米花和尺寸较小(40 nm)的多元醇包覆的ZnO NP。通过荧光硫黄素T(ThT)测量,监测了牛血清白蛋白(BSA)和人胰岛素(HI)中纳米结构的不同形态对原纤化/抗原纤化过程的影响。尽管两种纳米材料都会影响淀粉样蛋白的形成机理及其分解,带有尖锐边缘的ZnO纳米花在两种模型蛋白中均表现出最大的淀粉样蛋白降解率(分别为73%和35%),并最大程度地抑制了胰岛素原纤维的生长,同时在白蛋白溶液中也抑制了原纤化过程。在计算机上对BSA和HI的晶体结构进行分子对接模拟,以进一步分析观察到的ZnO纳米结构的体外活性。发现ZnO NPs的结合能对BSA较低(-5.44),突显了它们在白蛋白单体的原纤化过程中充当催化剂的能力。在计算机上对BSA和HI的晶体结构进行分子对接模拟,以进一步分析观察到的ZnO纳米结构的体外活性。发现ZnO NPs的结合能对BSA较低(-5.44),突显了它们在白蛋白单体原纤化过程中作为催化剂的能力。在计算机上对BSA和HI的晶体结构进行分子对接模拟,以进一步分析观察到的ZnO纳米结构的体外活性。发现ZnO NPs的结合能对BSA较低(-5.44),突显了它们在白蛋白单体的原纤化过程中充当催化剂的能力。
更新日期:2020-01-13
中文翻译:

ZnO纳米结构与蛋白质的相互作用:体外原纤化/抗原纤化研究和计算机分子对接模拟。
蛋白质淀粉样变性病与许多神经系统疾病有关。纳米颗粒(NPs)由于其尺寸小,可以调节多肽单体/寡聚体组装成淀粉样原纤维/斑块以及现有斑块的崩解。在这里,我们分别通过溶剂热和微波辅助途径合成了圆柱形的ZnO纳米花和尺寸较小(40 nm)的多元醇包覆的ZnO NP。通过荧光硫黄素T(ThT)测量,监测了牛血清白蛋白(BSA)和人胰岛素(HI)中纳米结构的不同形态对原纤化/抗原纤化过程的影响。尽管两种纳米材料都会影响淀粉样蛋白的形成机理及其分解,带有尖锐边缘的ZnO纳米花在两种模型蛋白中均表现出最大的淀粉样蛋白降解率(分别为73%和35%),并最大程度地抑制了胰岛素原纤维的生长,同时在白蛋白溶液中也抑制了原纤化过程。在计算机上对BSA和HI的晶体结构进行分子对接模拟,以进一步分析观察到的ZnO纳米结构的体外活性。发现ZnO NPs的结合能对BSA较低(-5.44),突显了它们在白蛋白单体的原纤化过程中充当催化剂的能力。在计算机上对BSA和HI的晶体结构进行分子对接模拟,以进一步分析观察到的ZnO纳米结构的体外活性。发现ZnO NPs的结合能对BSA较低(-5.44),突显了它们在白蛋白单体原纤化过程中作为催化剂的能力。在计算机上对BSA和HI的晶体结构进行分子对接模拟,以进一步分析观察到的ZnO纳米结构的体外活性。发现ZnO NPs的结合能对BSA较低(-5.44),突显了它们在白蛋白单体的原纤化过程中充当催化剂的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号