当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effective delivery of Complex Innovative Design (CID) cancer trials-A consensus statement.
British Journal of Cancer ( IF 6.4 ) Pub Date : 2020-01-06 , DOI: 10.1038/s41416-019-0653-9 Sarah P Blagden 1 , Lucinda Billingham 2 , Louise C Brown 3 , Sean W Buckland 4 , Alison M Cooper 5 , Stephanie Ellis 6 , Wendy Fisher 7 , Helen Hughes 8 , Debbie A Keatley 9 , Francois M Maignen 10 , Alex Morozov 11 , Will Navaie 6 , Sarah Pearson 12 , Abeer Shaaban 13 , Kirsty Wydenbach 14 , Pamela R Kearns 2, 15 ,
British Journal of Cancer ( IF 6.4 ) Pub Date : 2020-01-06 , DOI: 10.1038/s41416-019-0653-9 Sarah P Blagden 1 , Lucinda Billingham 2 , Louise C Brown 3 , Sean W Buckland 4 , Alison M Cooper 5 , Stephanie Ellis 6 , Wendy Fisher 7 , Helen Hughes 8 , Debbie A Keatley 9 , Francois M Maignen 10 , Alex Morozov 11 , Will Navaie 6 , Sarah Pearson 12 , Abeer Shaaban 13 , Kirsty Wydenbach 14 , Pamela R Kearns 2, 15 ,
Affiliation
|
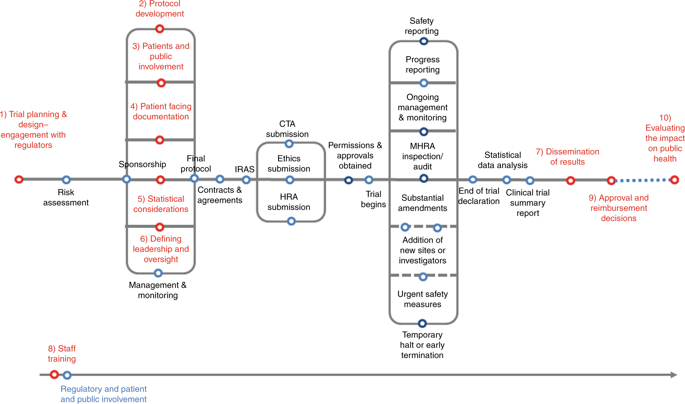
The traditional cancer drug development pathway is increasingly being superseded by trials that address multiple clinical questions. These are collectively termed Complex Innovative Design (CID) trials. CID trials not only assess the safety and toxicity of novel anticancer medicines but also their efficacy in biomarker-selected patients, specific cancer cohorts or in combination with other agents. They can be adapted to include new cohorts and test additional agents within a single protocol. Whilst CID trials can speed up the traditional route to drug licencing, they can be challenging to design, conduct and interpret. The Experimental Cancer Medicine Centres (ECMC) network, funded by the National Institute for Health Research (NIHR), Cancer Research UK (CRUK) and the Health Boards of Wales, Northern Ireland and Scotland, formed a working group with relevant stakeholders from clinical trials units, the pharmaceutical industry, funding bodies, regulators and patients to identify the main challenges of CID trials. The working group generated ten consensus recommendations. These aim to improve the conduct, quality and acceptability of oncology CID trials in clinical research and, importantly, to expedite the process by which effective treatments can reach cancer patients.
中文翻译:

有效实施复杂创新设计 (CID) 癌症试验 - 共识声明。
传统的癌症药物开发途径越来越多地被解决多个临床问题的试验所取代。这些统称为复杂创新设计(CID)试验。 CID 试验不仅评估新型抗癌药物的安全性和毒性,还评估其对生物标志物选择的患者、特定癌症群体或与其他药物联合使用的疗效。它们可以进行调整,以纳入新的队列并在单一方案中测试其他药物。虽然 CID 试验可以加快传统的药品许可途径,但它们的设计、实施和解释可能具有挑战性。实验癌症医学中心 (ECMC) 网络由国家健康研究所 (NIHR)、英国癌症研究中心 (CRUK) 以及威尔士、北爱尔兰和苏格兰卫生委员会资助,与临床试验的相关利益相关者组成了一个工作组单位、制药行业、资助机构、监管机构和患者,以确定 CID 试验的主要挑战。工作组提出了十项共识建议。这些旨在改善临床研究中肿瘤学 CID 试验的实施、质量和可接受性,更重要的是,加快癌症患者获得有效治疗的进程。
更新日期:2020-01-06
中文翻译:

有效实施复杂创新设计 (CID) 癌症试验 - 共识声明。
传统的癌症药物开发途径越来越多地被解决多个临床问题的试验所取代。这些统称为复杂创新设计(CID)试验。 CID 试验不仅评估新型抗癌药物的安全性和毒性,还评估其对生物标志物选择的患者、特定癌症群体或与其他药物联合使用的疗效。它们可以进行调整,以纳入新的队列并在单一方案中测试其他药物。虽然 CID 试验可以加快传统的药品许可途径,但它们的设计、实施和解释可能具有挑战性。实验癌症医学中心 (ECMC) 网络由国家健康研究所 (NIHR)、英国癌症研究中心 (CRUK) 以及威尔士、北爱尔兰和苏格兰卫生委员会资助,与临床试验的相关利益相关者组成了一个工作组单位、制药行业、资助机构、监管机构和患者,以确定 CID 试验的主要挑战。工作组提出了十项共识建议。这些旨在改善临床研究中肿瘤学 CID 试验的实施、质量和可接受性,更重要的是,加快癌症患者获得有效治疗的进程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号