Nature Chemical Biology ( IF 12.9 ) Pub Date : 2020-01-06 , DOI: 10.1038/s41589-019-0435-y Abhishek A Jalan 1, 2 , Douglas Sammon 3 , Jeffrey D Hartgerink 4 , Paul Brear 1 , Katherine Stott 1 , Samir W Hamaia 1 , Emma J Hunter 1 , Douglas R Walker 4 , Birgit Leitinger 3 , Richard W Farndale 1
|
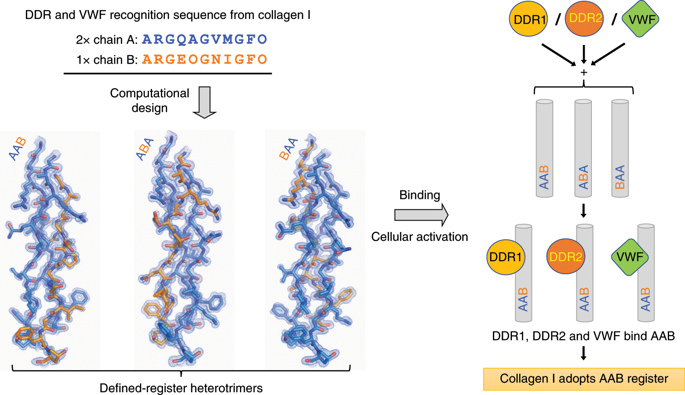
The most abundant member of the collagen protein family, collagen I (also known as type I collagen; COL1), is composed of one unique (chain B) and two similar (chain A) polypeptides that self-assemble with one amino acid offset into a heterotrimeric triple helix. Given the offset, chain B can occupy either the leading (BAA), middle (ABA) or trailing (AAB) position of the triple helix, yielding three isomeric biomacromolecules with different protein recognition properties. Despite five decades of intensive research, there is no consensus on the position of chain B in COL1. Here, three triple-helical heterotrimers that each contain a putative von Willebrand factor (VWF) and discoidin domain receptor (DDR) recognition sequence from COL1 were designed with chain B permutated in all three positions. AAB demonstrated a strong preference for both VWF and DDR, and also induced higher levels of cellular DDR phosphorylation. Thus, we resolve this long-standing mystery and show that COL1 adopts an AAB register.
中文翻译:

使用计算设计的异源三聚体破译的胶原蛋白链排列
胶原蛋白家族中最丰富的成员,胶原蛋白 I(也称为 I 型胶原蛋白;COL1)由一个独特的(链 B)和两个相似的(链 A)多肽组成,它们通过一个氨基酸偏移自组装成异三聚体三螺旋。鉴于偏移,链 B 可以占据三螺旋的前导 (BAA)、中间 (ABA) 或尾随 (AAB) 位置,从而产生具有不同蛋白质识别特性的三种异构生物大分子。尽管经过 5 年的深入研究,B 链在 COL1 中的位置并没有达成共识。在这里,设计了三个三螺旋异源三聚体,每个三聚体均包含来自 COL1 的推定的血管性血友病因子 (VWF) 和盘状结构域受体 (DDR) 识别序列,其中 B 链在所有三个位置均排列。AAB 表现出对 VWF 和 DDR 的强烈偏好,并且还诱导了更高水平的细胞 DDR 磷酸化。因此,我们解决了这个长期存在的谜团,并表明 COL1 采用了 AAB 寄存器。











































 京公网安备 11010802027423号
京公网安备 11010802027423号